Apoptosis is a controlled process of cell death, involving progressive shrinkage and formation of apoptotic bodies, small fragments of cells with an intact membrane. Because the membrane remains intact, apoptosis is not associated with inflammation, unlike necrosis.
Table of Contents
Pathogenesis
There are three main pathways in apoptosis: intrinsic, extrinsic and perforin/granzyme. All of the pathways are mediated through proteins called caspases.
Intrinsic Pathway
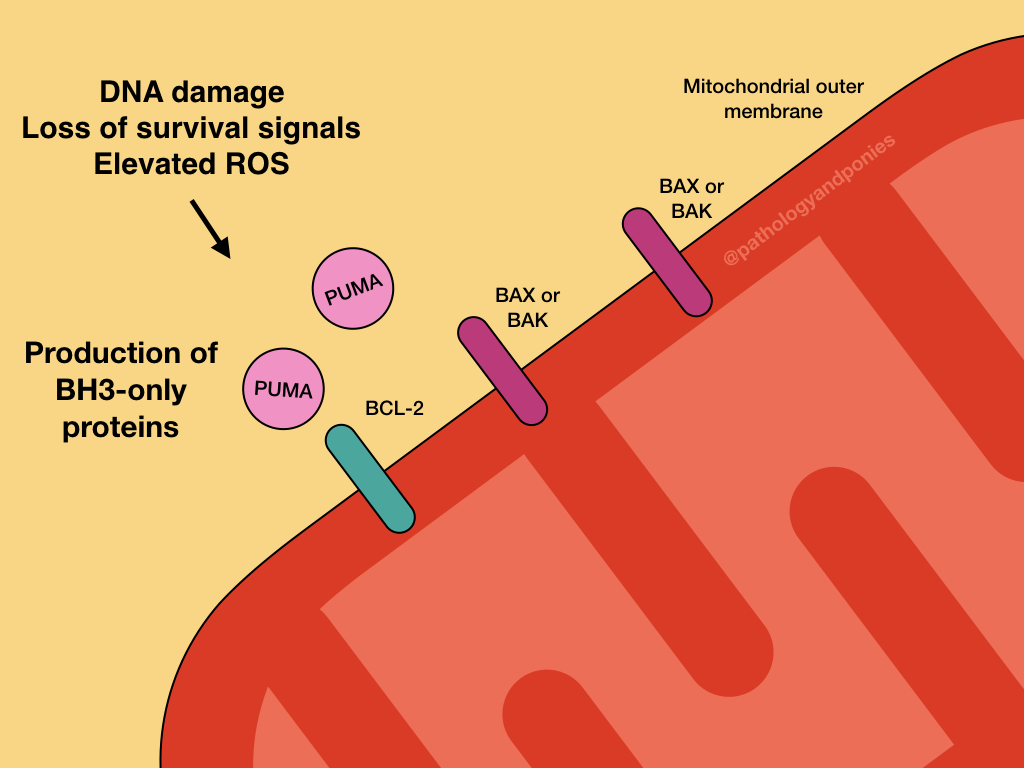
The intrinsic pathway responds to problems within the cell, like toxins, reactive oxygen species and DNA damage. These problems result in activation of p53-upregulated modulator of apoptosis (PUMA), and other regulatory BCL proteins called BH3-only proteins.
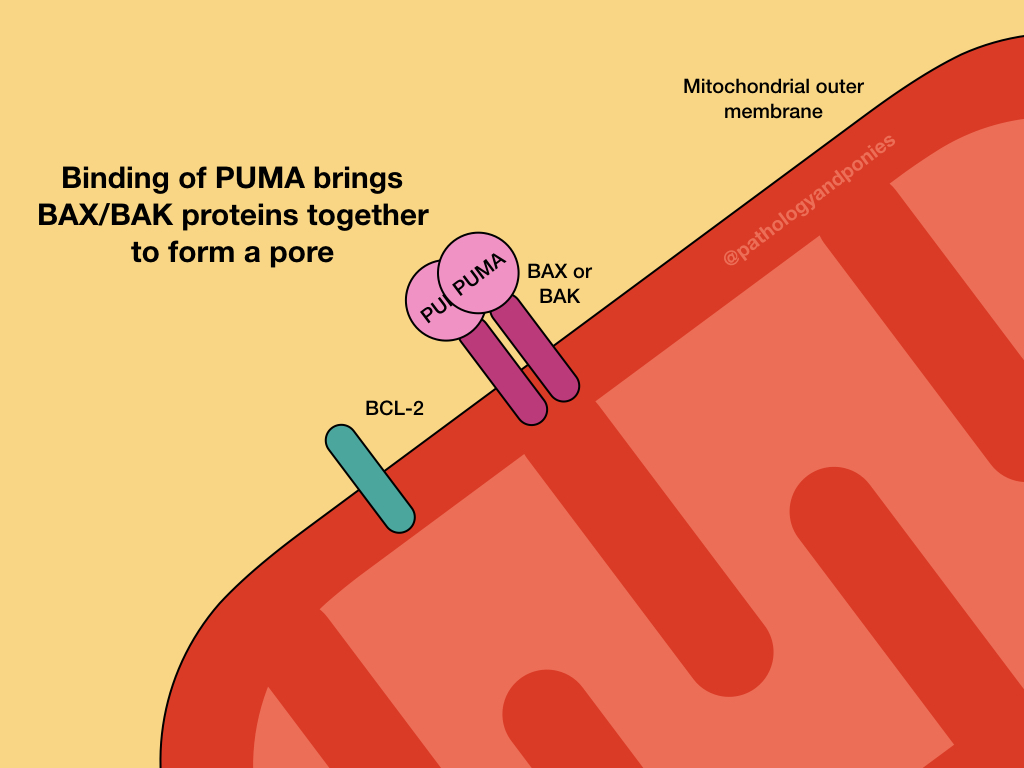
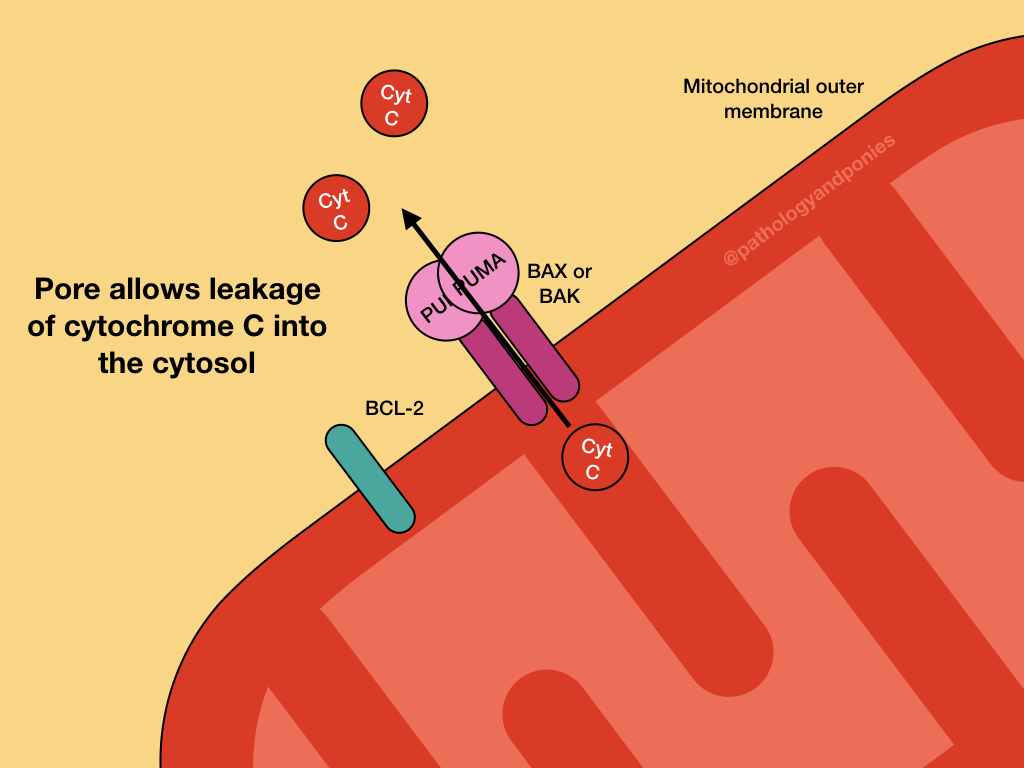
PUMA and the other BH3-only regulatory proteins activate BAX and BAK, which form oligomers on the outer mitochondrial membrane. This results in the formation of channels in a process called mitochondrial outer membrane permeabilization (MOMP).
Opening of the channels results in protein release into the cytosol. The most important of these proteins is cytochrome C, which binds to apoptosis-activating factor-1 (APAF-1) to form the apoptosome. The apoptosome activates caspase-9, which is the major initiator caspase for intrinsic apoptosis. Executor caspases are activated, ultimately resulting in apoptosis.
Other notable proteins released from MOMP include Smac and DIABLO, which bind to and inhibit inhibitors of apoptosis (IAP) proteins. These IAPs normally prevent activation of caspases, so their inhibition by Smac and DIABLO pushes the cell closer to apoptosis.
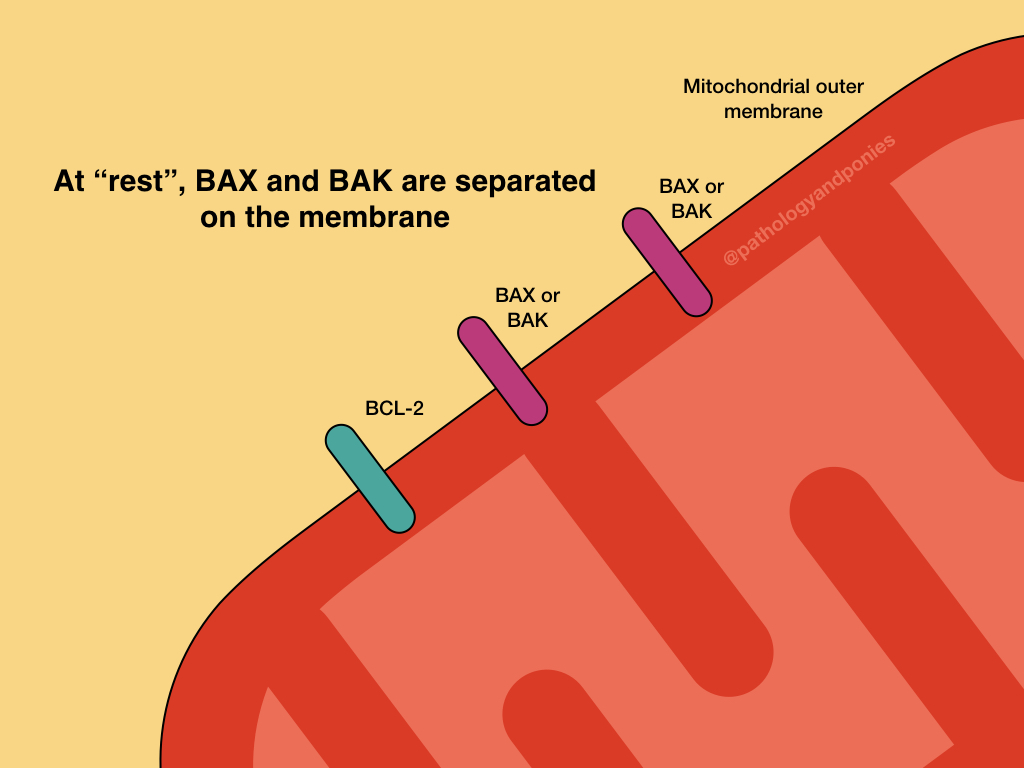
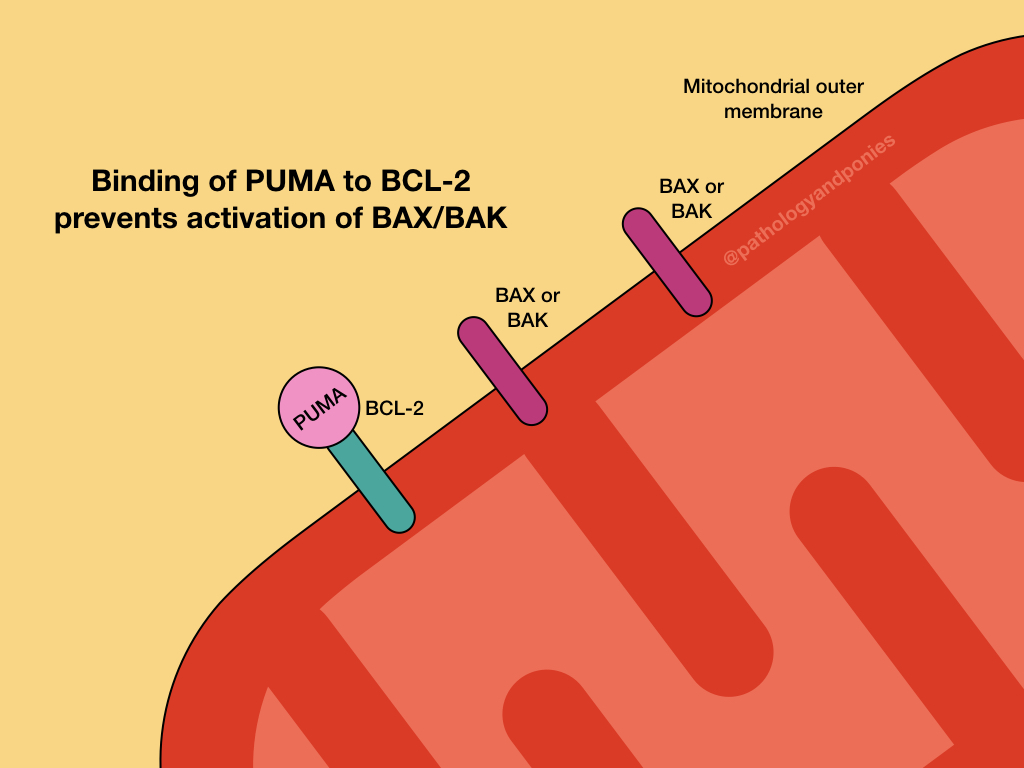
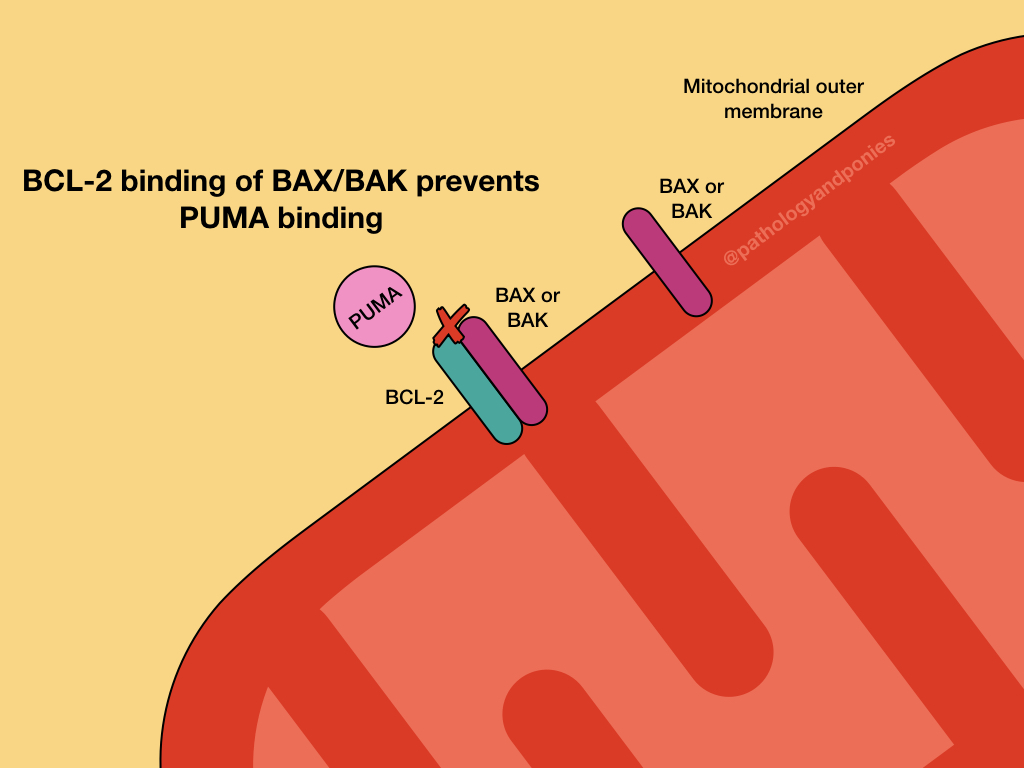
The intrinsic pathway is prevented by upregulation of the anti-apoptotic BCL proteins, BCL2 and BCL-XL. These proteins serve to bind BH3-only regulatory proteins to prevent them from activating BAX and BAK. They can also bind BAX and BAK directly to prevent their activation.
Extrinsic Pathway
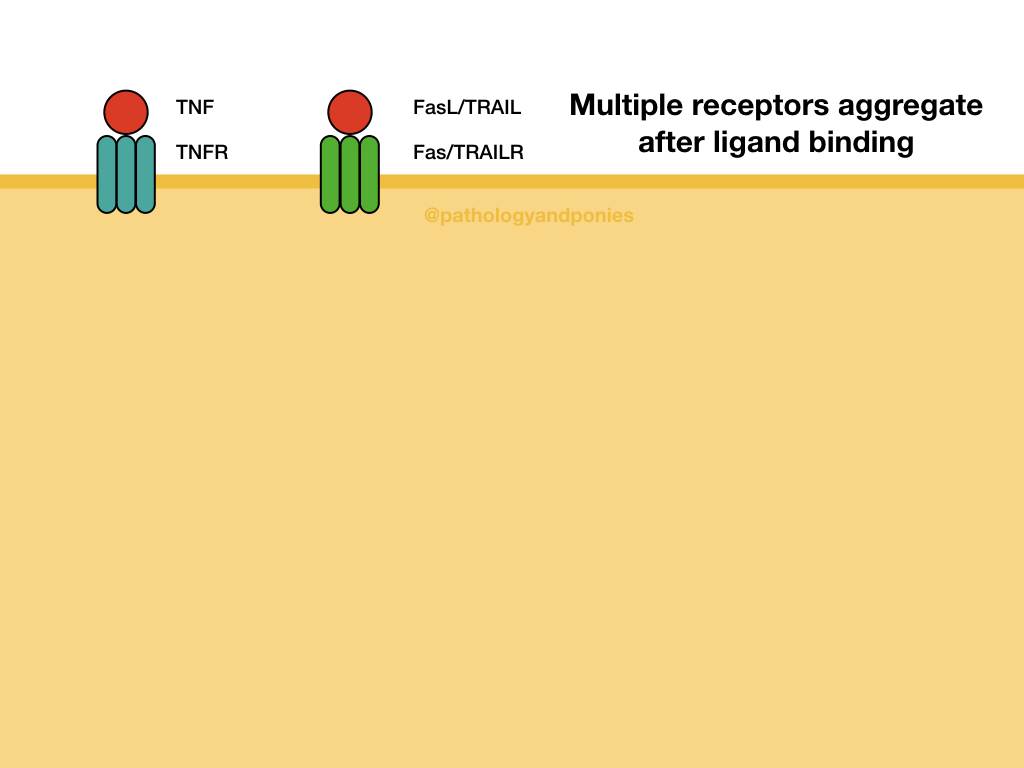
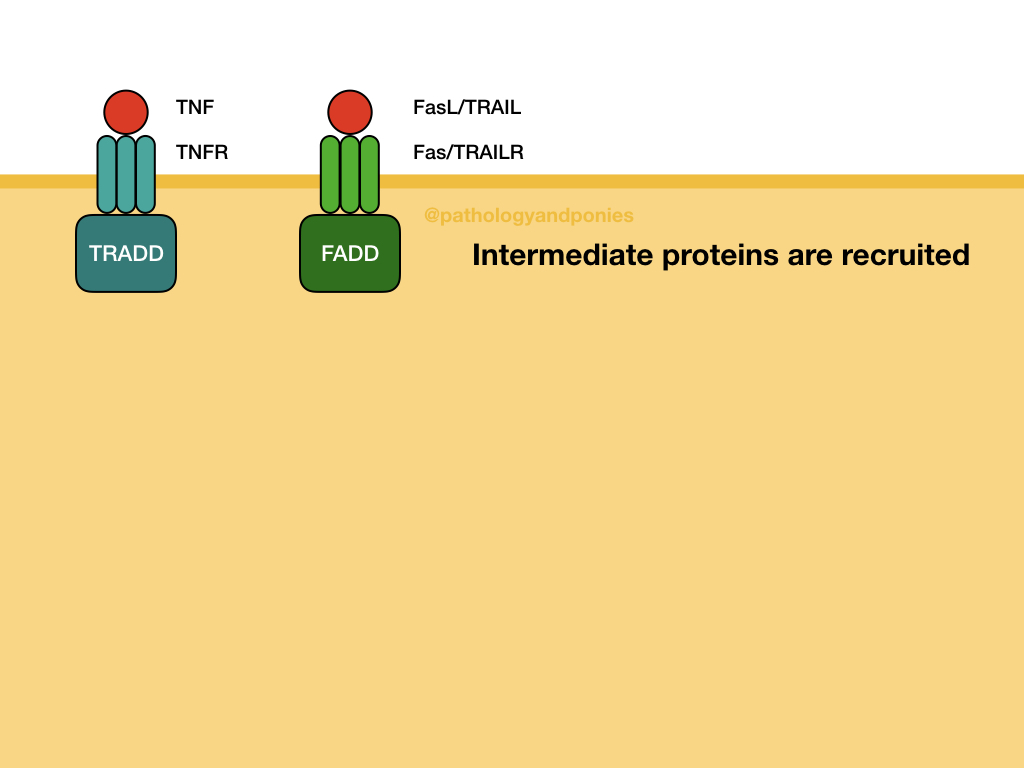
The extrinsic pathway responds to external factors, such as ligands binding to death receptors on the cell surface. Example death receptors include Fas, TNF receptor, and TRAILR. After the ligand binds, the receptor is internalized and associates with receptor-specific intermediate proteins. For example, Fas and TRAILR bind to Fas-associated death domain (FADD), and TNF binds TNF-receptor associated death domain (TRADD).
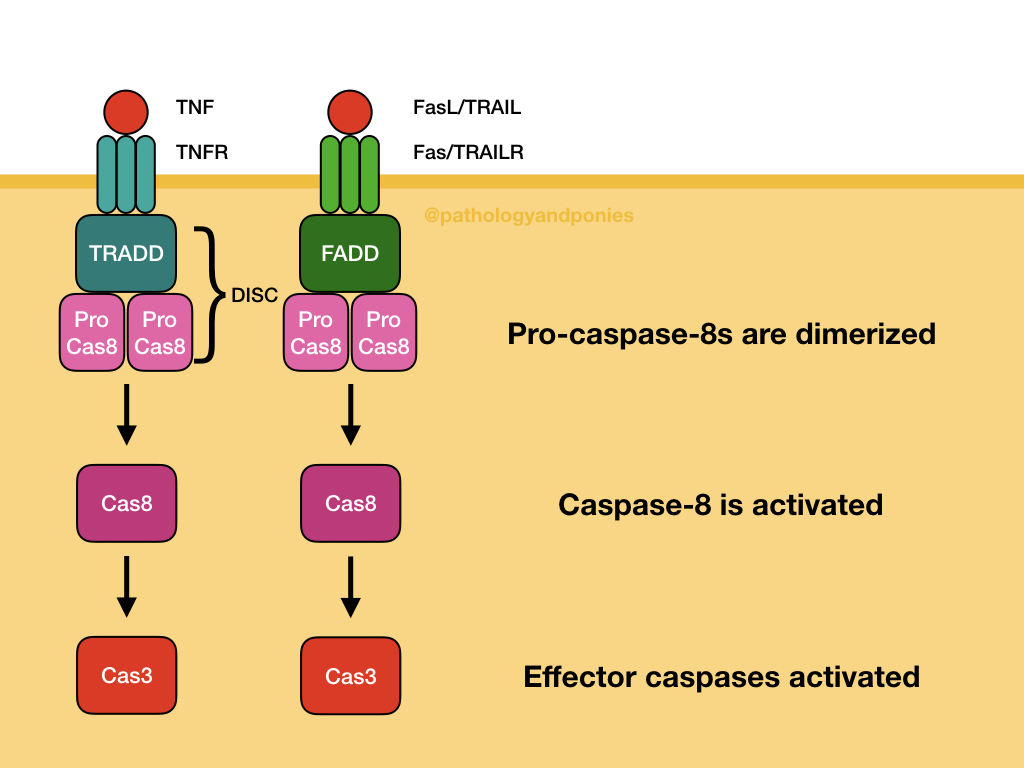
These intracellular proteins combine with pro-caspase-8, the precursor to the effector caspase of the extrinsic pathway. Multiple pro-caspase-8s are combined to generate caspase-8. These protein groups are called the death-inducing signaling complex (DISC). From there, caspase-8 activates executioner caspases, leading to apoptosis.
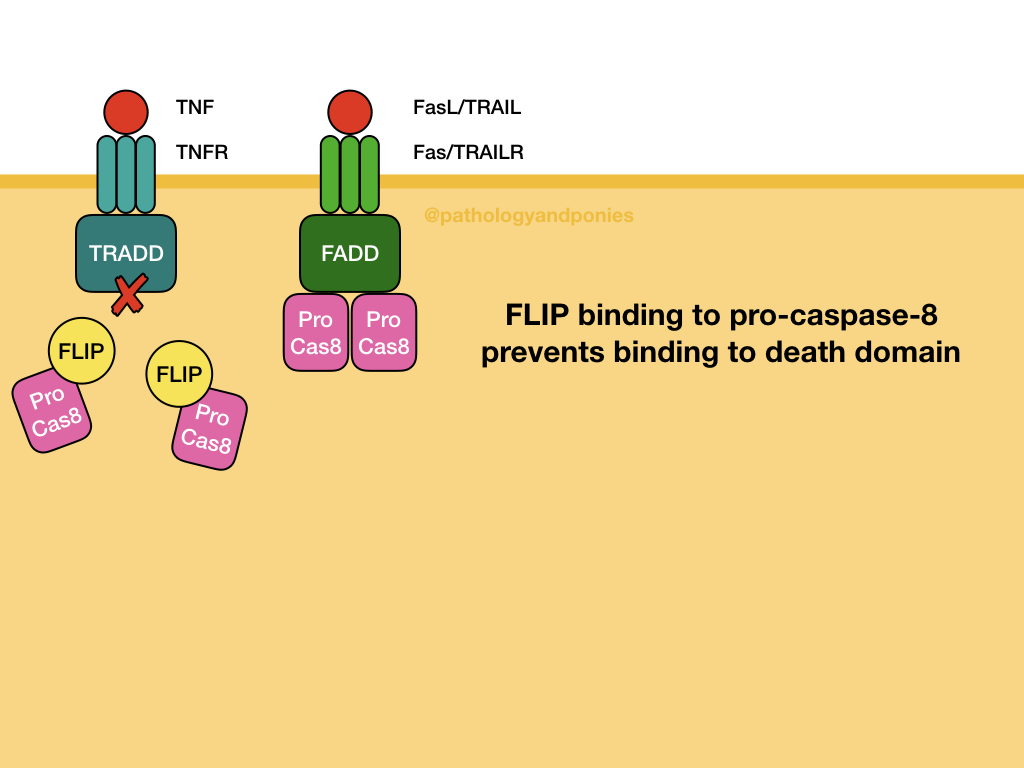
The main inhibitor of the extrinsic pathway is FLIP protein, which binds pro-caspase-8. This binding prevents death domains from activating the caspase, and prevents extrinsic apoptosis.
A brief note on anoikis:
Anoikis, or loss of cell contact with the basement membrane, can induce apoptosis through both the extrinsic and intrinsic pathways.
Perforin/Granzyme Pathway
Perforin and granzyme come from cytotoxic T cells and natural killer cells, and serve to induce apoptosis in cells with intracellular pathogens or neoplastic cells. Perforin forms a pore in the cell membrane that allows granzyme to enter into the cytosol. From there, granzyme activates caspase-10, which activates executioner caspases.
Execution Phase
All of the above caspases result in activation of executioner caspases, which ultimately allow apoptosis to proceed. The main executioner caspases are caspase-3 and caspase-6. They act as inhibitors of DNase, allowing degradation of DNA. They can also proteolytically cleave nucleus components. It is suspected that these caspases are involved in formation of membrane blebs and apoptotic bodies, however the exact mechanism is unknown.
Clean-Up Phase
The end result of apoptosis is formation of apoptotic bodies, which need to be removed from the tissue. These apoptotic bodies can target themselves for phagocytosis by flipping phosphotidylserine to the outside of their membrane for recognition by macrophages. They can also become coated with C1q complement proteins for opsonization.
Cell Morphology
The hallmarks of apoptosis under the microscope are:
- Pyknosis and karyorrhexis from DNase activity.
- Blebbing of the membrane and formation of apoptotic bodies.
Zachary JF. Pathologic Basis of Veterinary Disease, Sixth Edition.
Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease, Tenth Edition.