Immunodeficiency is caused by lack of function in one or more components of the immune system, preventing an appropriate immune response by the animal. These conditions lead to an increased risk of infections and potentially an increased risk of tumour development. Immunodeficiency is either primary, caused by congenital or genetic issues, or secondary, due to damage or malfunction by toxins, infections, nutritional deficiencies, etc.
Primary Immunodeficiency
While there are many primary immunodeficiencies described in humans, there are only a few notable ones in veterinary species.
Severe Combined Immunodeficiency Disease
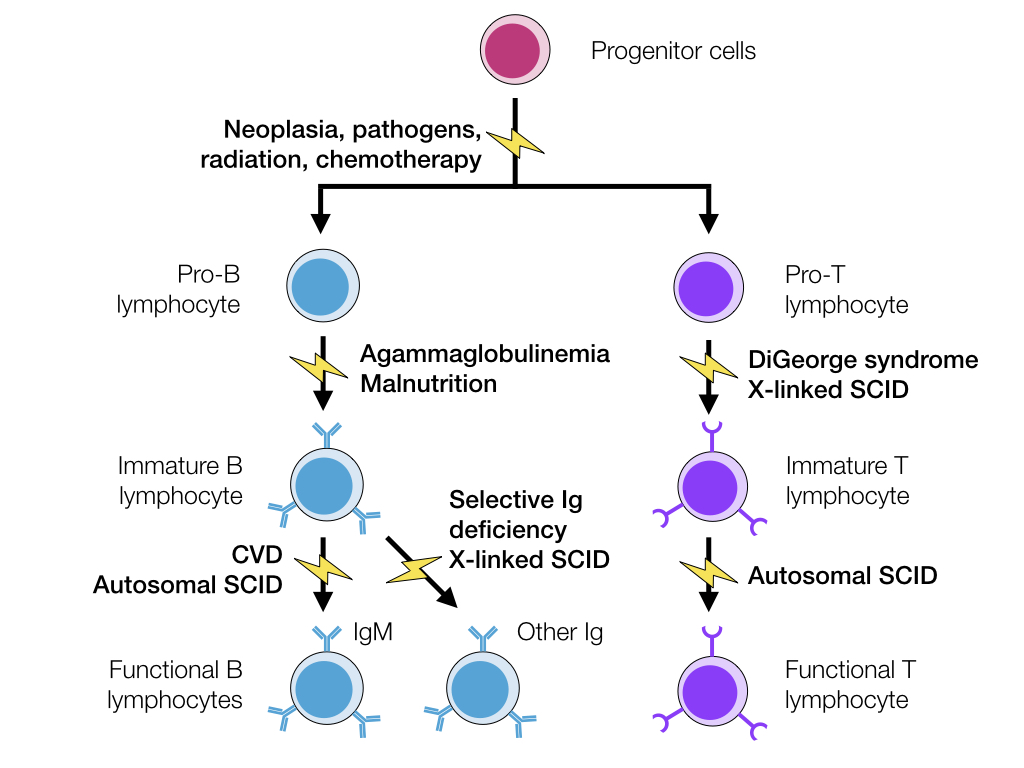
SCID is a genetic defect that can affect both T and B lymphocytes, or may affect just T lymphocytes, depending on the cause. In cases with just T lymphocyte defects, B lymphocytes will also have reduced function due to reduced signalling for B cell activation. In SCID, the animal is at an increased risk of fungal and viral infections due to decreased T cell function, and at an increased risk of bacterial infection from decreased B cell function.
The genetic defect causing SCID depends on the species:
| Animal | Mode of Inheritance | Genetic Defect | Outcome |
|---|---|---|---|
| Arabian horses Mice Jack Russells | Autosomal recessive | DNA-dependent protein kinase | Failure of recombination of the binding site of T-cell receptors and immunoglobulins |
| Many dog breeds | X-linked | Mutation in ɣ subunit of type I cytokines like IL-2 | Nonfunctional T lymphocytes Incapable of class-switching to IgG |
Animals with SCID often have a small thymus with no corticomedullary differentiation, very few lymphocytes and minimal Hassall’s corpuscles. In the spleen and lymph nodes, there are no lymphoid follicles or plasma cells.
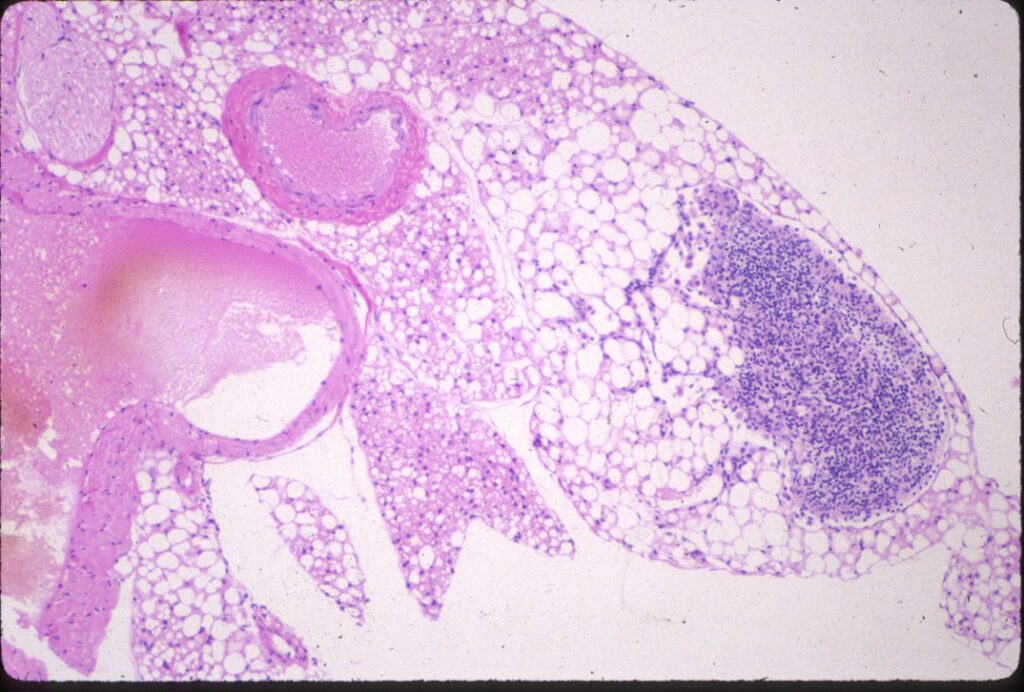
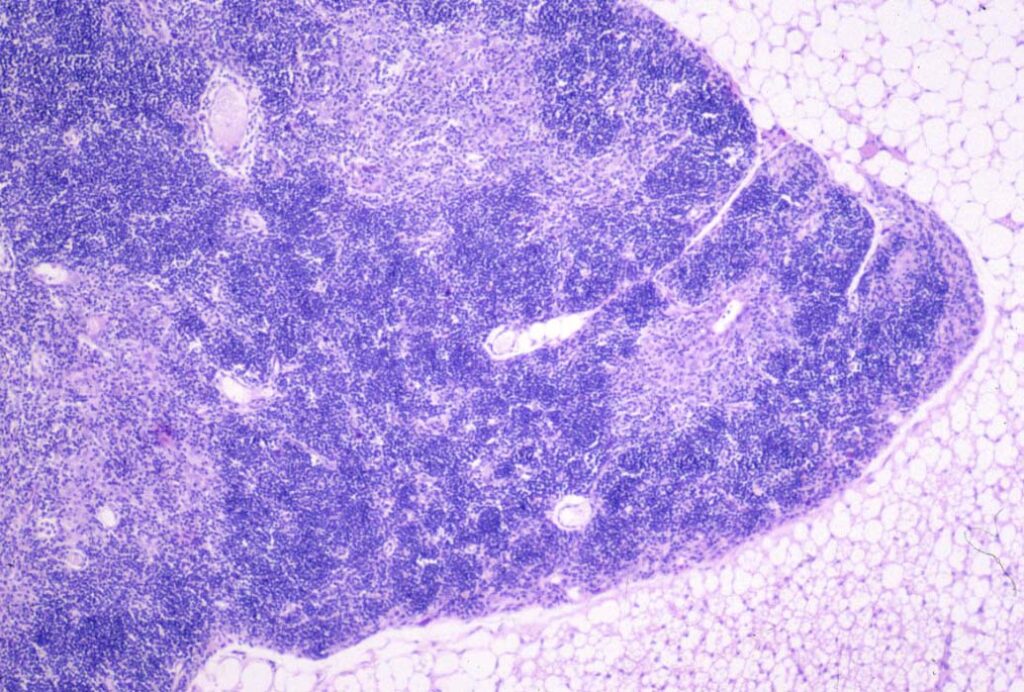
© Sundberg licensed under CC BY-SA 4.0.
Common Variable Deficiency
CVD is caused by a defect in B lymphocytes, leading to few to no B lymphocytes in the bone marrow and spleen and no immunoglobulins in circulation. In humans, CVD can be caused by many different mutations, hence the name “variable” deficiency.
Agammaglobulinemia
Agammaglobulinemia is when the animal is unable to produce immunoglobulins. In humans, this condition is caused by a genetic mutation that stops lymphocyte development before they mature. Affected animals have few to no plasma cells and lack lymphoid follicles.
Immunoglobulin Deficiencies
Some genetic mutations can cause deficiency of specific immunoglobulins, such as IgM deficiency or IgA deficiency. In these conditions, there are normal levels of B lymphocytes and other types of immunoglobulins, but the specific affected immunoglobulin is deficient. IgM deficiency has been described in horses, and has been associated with recurring respiratory infections. IgA deficiency has primarily been described in dogs. Since IgA primarily contributes to mucosal immunity, this deficiency results in recurring infections of mucosal surfaces including the skin.
Thymic Hypoplasia
Thymic hypoplasia is called DiGeorge syndrome in humans, and is characterized by a non-functional thymus with secondary T lymphocyte deficiency. “Nude mice” are the main animal model of this disease, and are used in research for testing organ transplants and other grafts. The human syndrome is thought to be due to an embryologic defect preventing proper development of the thymus from the pharyngeal pouches. Histologically, the periarteriolar sheaths of the spleen and the paracortical areas of lymph nodes are depleted.
Secondary Immunodeficiency
Secondary immunodeficiency is acquired during the animal’s life, and is usually due to some type of insult to the immune system. Some examples are included below:
- Pathogenic insults depleting lymphocytes, such as feline immunodeficiency virus.
- Radiation therapy and chemotherapy during cancer treatment decreasing bone marrow precursors.
- Bone marrow neoplasia from metastasis, etc. reducing the available area for leukocyte development.
- Malnutrition preventing formation of immunoglobulins due to lack of protein.
Non-specific Immunodeficiency
The non-specific components of the immune system can be affected by primary immunodeficiencies as well. Some important ones are included below.
Complement Deficiency
Because the complement system has so many involved proteins, mutation resulting in complement deficiency is bound to happen.
Most complement mutations affect C2, which is a major component of the classical pathway. Affected individuals do not seem to be at an increased risk of infection, likely due to activation of the alternative or lectin pathways instead. However, classical pathway deficiencies may be linked to autoimmune disease secondary to poor clearance of antigen-antibody complexes. Alternative pathway deficiencies are associated with an increased risk of bacterial infections.
C3 deficiency is the most significant complement deficiency, as all three complement pathways require its activation to allow progress. This deficiency has been described in dogs, and predisposes them to severe bacterial infections.
Factor H deficiency has been described in Norwegian Yorkshire pigs, and is sometimes called porcine dense deposit disease. This is because Factor H is normally involved in cleavage of C3b. Without Factor H, C3b remains in circulation, where it deposits in the kidneys causing glomerulonephritis. Humans with Factor H deficiency are also predisposed to bacterial infections.
It is also possible for the proteins composing the MAC complex (C5, C6, C7, C8 and C9) to be deficient. Similar to C3 deficiency, this disrupts all three complement pathways, leading to severe bacterial infections.
Chédiak-Higashi Syndrome
Chédiak-Higashi syndrome has been described in many veterinary species, even killer whales! This disease affects lysosomes and other storage granules within cells, preventing them from removing their stored materials. This syndrome affects all cells, so these animals may have hypopigmentation due to inadequate melanocyte granule movement, coagulopathies due to poor platelet granule release, etc. In humans, this condition is associated with mutation of the Lyst gene, which regulates intracellular protein trafficking.
This syndrome affects the immune system because neutrophils cannot use their granules appropriately for directing intracellular killing. Similarly, they may not receive adequate signalling for chemotaxis if the signalling molecules cannot be released from granules. Natural killer cells are also defective in this condition. These combined effects increase the animal’s susceptibility to infections, along with the other consequences of decreased granule function noted earlier.
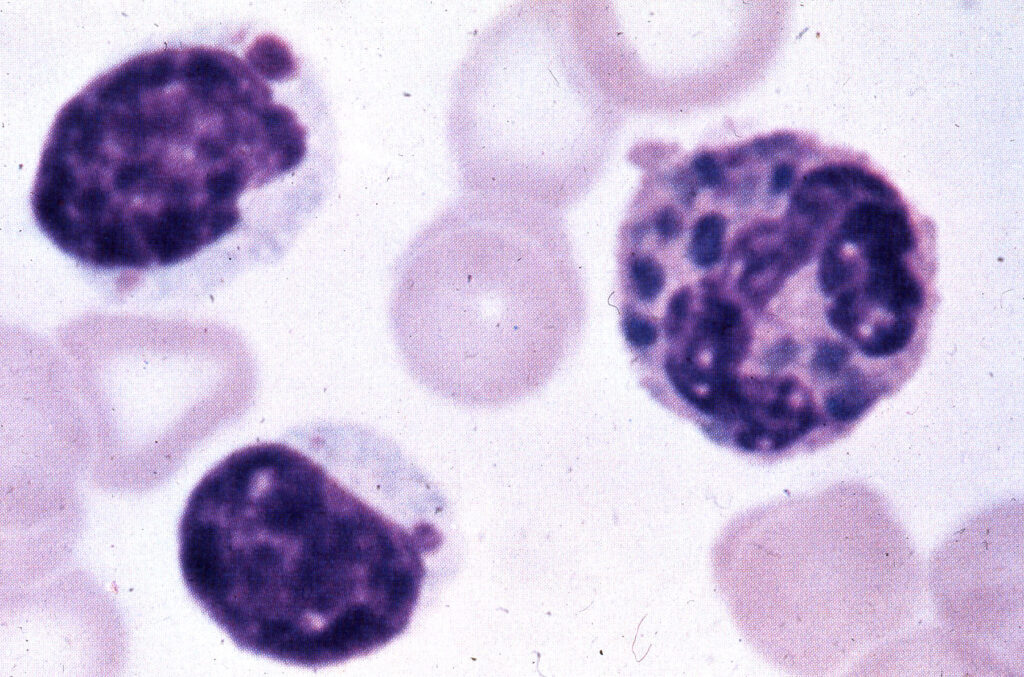
© Jakowksi licensed under CC BY-SA 4.0.
Leukocyte Adhesion Deficiency
This name is pretty self-explanatory! In this condition, there is a deficit in the adhesion molecules that normally allow leukocyte adhesion to endothelium during inflammation. Without proper leukocyte migration into the tissue, bacterial infections are not properly cleared.
Zachary JF. Pathologic Basis of Veterinary Disease, Sixth Edition.
Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease, Tenth Edition.