Cortisol is probably the most well known of the glucocorticoids, which are produced by the zona fasciculata of the adrenal cortex. Its main functions are increasing blood sugar by increasing glucose synthesis, suppression of the immune system, and metabolism of other energy sources like fats, proteins and carbohydrates. Because it has such varying effects, having a dysfunction in cortisol levels can be very significant for the animal!
In order for the adrenal cortex to release cortisol, it needs to be stimulated. This occurs via the hypothalamic-pituitary-adrenal axis. In this series of steps, the hypothalamus first releases corticotropin-releasing hormone, which acts on the anterior pituitary gland to secrete adrenocorticotropic hormone (ACTH) into the bloodstream. This hormone ultimately reaches the adrenal cortex, and stimulates synthesis and release of cortisol. Any step along this pathway is a potential site for cortisol dysfunction to arise!
Table of Contents
Hyperadrenocorticism
As mentioned, any step along the cortisol production pathway could lead to cortisol dysfunction. Hyperadrenocorticism, or Cushing syndrome, is a great example of that! There are two basic types: pituitary-dependent and adrenal-dependent. This condition can also be caused iatrogenically, if the animal is administered excessive steroids. Typically, hyperadrenocorticism is seen in middle-aged and older dogs.
Pituitary-Dependent Hyperadrenocorticism
Pituitary-dependent hyperadrenocorticism is by far the most common cause of hyperadrenocorticism, with about 85% of dogs with Cushing syndrome having this type. This type of hyperadrenocorticism is typically caused by adenomas of the pituitary gland that are functional, and produce high amounts of corticotropin-releasing hormone. High levels of this hormone ultimately acts on the adrenal cortex to produce elevated levels of cortisol.

© King licensed under CC BY-SA 4.0.
Adrenal-Dependent Hyperadrenocorticism
Adrenal-dependent hyperadrenocorticism is less common, and is caused by adrenocortical adenomas or carcinomas. These tumours are functional, and secrete cortisol on their own without any pituitary stimulation.
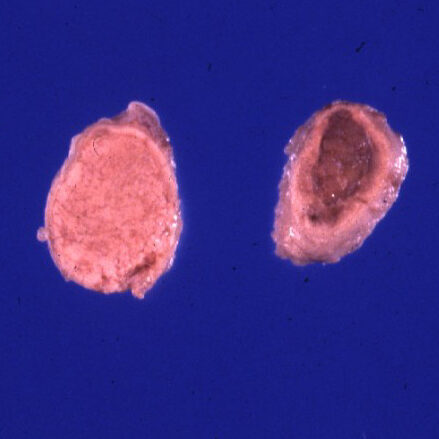
© Wright licensed under CC BY-SA 4.0.
Clinical Signs
- Polydipsia and polyuria. This is because cortisol has a negative feedback effect on antidiuretic hormone, which normally acts on the kidney to reduce fluid excretion, and urination.
- Abdominal enlargement, specifically a “pot-bellied” appearance. Elevated cortisol breaks down protein, including muscle, leading to weakening of the abdominal muscles. It can also cause general muscle weakness.
- Skin changes, including skin infections, hyperpigmentation, alopecia, skin exudates and bruising. A more uncommon, but classic, skin finding is calcinosis cutis, where deposits of calcium are found in the collagen of the skin.
- Elevated liver enzymes, due to the effects of steroid hepatopathy. Cortisol increases glycogen synthesis in the liver, resulting in liver cells that are severely distended with glycogen. Ultimately, these aggregates of glycogen can interfere with normal liver function, leading to elevated liver enzymes.
- Hypercholesterolemia, due to cortisol stimulating breakdown of fat.
A brief note on cats:
Cats can also present with very thin, fragile skin from hyperadrenocorticism. Sometimes, this presents as multiple self-inflicted wounds, or even incidents where large portions of the cat’s skin tears off. Horrifying!
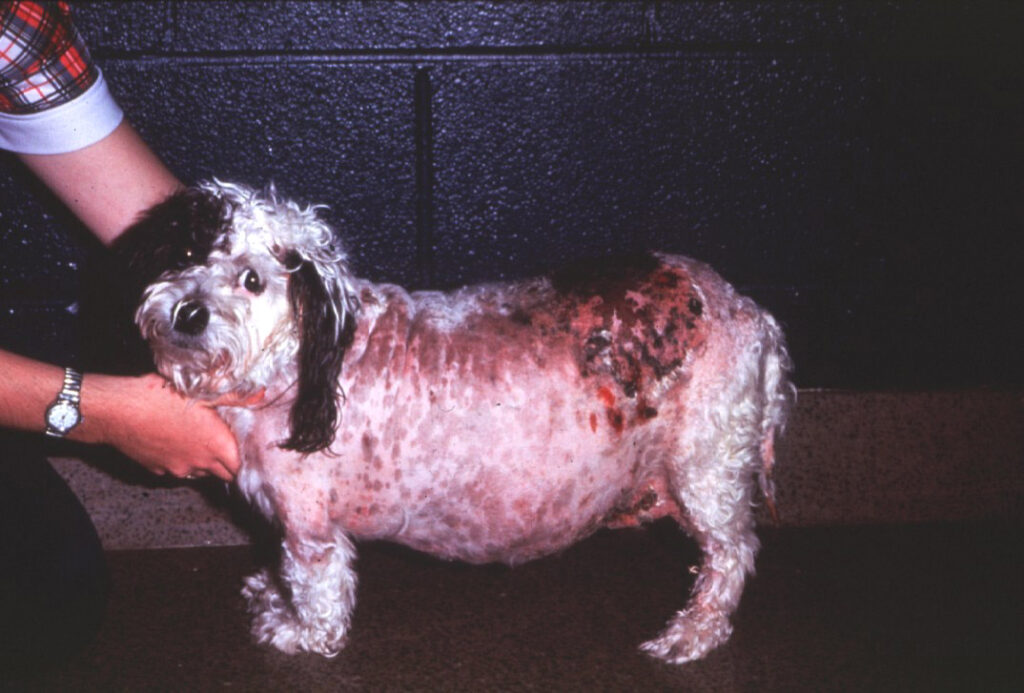
© Barsanti licensed under CC BY-SA 4.0.

© Harrington licensed under CC BY-SA 4.0.
Diagnosis
Hyperadrenocorticism diagnosis seems a bit complicated at first, but hopefully I can help make it clear!
The first test you want to run in a suspect case is a low-dose dexamethasone suppression test (LDDS). This test works by administering a small amount of dexamethasone, which is another glucocorticoid. Because it has a similar function to cortisol, the dexamethasone will provide negative feedback on the pituitary, to reduce release of ACTH.
Following dexamethasone administration, blood will be drawn and tested for cortisol levels at 4 and 8 hours, and compared to the baseline sample. This is where things start to get complicated!
If the animal has pituitary-dependent hyperadrenocorticism, i.e. it has a functional pituitary tumour, that tumour may still be somewhat responsive to the negative feedback loop of dexamethasone. However, it also may completely ignore the negative feedback loop, or the animal may have adrenal-dependent hypercorticism, in which case the amount of ACTH produced doesn’t matter. Here’s what these scenarios might look like on an LDDS:
| Type of HAC | Baseline Cortisol | 4 hours | 8 hours |
|---|---|---|---|
| Normal animal | Normal | Reduced | Reduced |
| Dex. responsive pituitary lesion | High | Reduced | High |
| Dex. unresponsive pituitary lesion | High | High | High |
| Adrenal lesion | High | High | High |
Interestingly, the responsive pituitary lesion pattern is considered to be diagnostic for pituitary-dependent hyperadrenocorticism, so if you see that pattern, your work is done! However, if we look at the results for an unresponsive pituitary lesion and an adrenal lesion, they are the same. This is why the LDDS test is considered a screening test, and cannot confirm adrenal-dependent vs. pituitary-dependent disease. So we need to do some further digging to help sort these two scenarios out!
This is where the high-dose dexamethasone suppression test comes in. It works on a similar principle, however it takes it a step further by suggesting that extreme levels of dexamethasone will suppress the majority of pituitary lesions. However, it will still have no effect on adrenal lesions. The results of an HDDS might look like this:
| Type of HAC | Baseline Cortisol | 4 hours | 8 hours |
|---|---|---|---|
| Normal animal | Normal | Severely reduced | Severely reduced |
| Pituitary lesion | High | Reduced | Reduced or high |
| Adrenal lesion | High | High | High |
Using these two tests, you can generally get a good idea of what type of hyperadrenocorticism is occurring. If you wanted to really confirm it, then ultrasound of the adrenals or CT scan or MRI of the brain are your best options.
Treatment
You might be thinking, who cares whether it’s adrenal-dependent or pituitary-dependent? Your treatment plan cares! Generally, treatments for both types of hyperadrenocorticism focus on the adrenal gland, however the treatment used can be quite different.
For animals with pituitary-dependent disease, removal of the functional tumour surgically would be ideal. However, the pituitary is part of the brain, and brain surgery in animals isn’t often done! This is why our treatment protocols tend to focus on the more easily “accessible” adrenal glands.
In particular, pituitary-dependent disease is often treated with mitotane, a drug that induces necrosis of the adrenal cortex. Without having a fully functional adrenal cortex, the amount of cortisol production is reduced, allowing the animal to return to normal levels, even though the amount of ACTH produced is the same. Another option is trilostane, which is an adrenal enzyme inhibitor, and has fewer side effects than mitotane.
On the adrenal-dependent side, surgery to remove adrenal tumours is often indicated. However, this surgery is very risky, and often comes with a high risk of hypoadrenocorticism following adrenal tissue removal. Medical treatment with mitotane can be tried, however most adrenal tumours are not susceptible to mitotane’s effects.
Hypoadrenocorticism
The opposite of hyperadrenocorticism is hypoadrenocorticism, or Addison disease. This condition is typically see in young to middle-aged dogs. Unlike Cushing syndrome, this disease typically arises from effects on the adrenal cortex. Most cases of this disease are considered to be idiopathic, although it is strongly suspected that there is an immune-mediated component involved in the destruction of the adrenal cortex.
A brief note on aldosterone:
It is important to note that in this condition, the zona glomerulosa is affected as well. This leads to decreased productionn of aldosterone, which regulates serum levels of potassium, sodium and chloride. Potassium begins to accumulate, while sodium and chloride are lost in the urine.

Clinical Signs
- Acute circulatory collapse due to a progressive decrease in blood volume over time. This occurs due to the effects of reduced aldosterone. Specifically, loss of sodium and chloride into the urine pulls more water into the urine, reducing blood volume. These animals often have a hyperkalemia as well, which can cause bradycardia. The low heart rate can also contribute to circulatory collapse.
- Gastroenteritis, including large amounts of hemorrhagic feces. The exact pathogenesis of this hasn’t been determined.
© Ramos-Vara licensed under CC BY-SA 4.0.
Diagnosis
In order to diagnose hypoadrenocorticism, an ACTH stimulation test is used. In this test, a dose of ACTH is administered to the animal, and cortisol concentrations are measured at 1-2 hours later. In a normal animal, ACTH administration would stimulate increased cortisol concentrations. An example below:
| Disease State | Baseline Cortisol | 2 hours |
|---|---|---|
| Normal animal | Normal | Elevated |
| Addisonian animal | Low | Low |
Finding low cortisol levels after ACTH administration is considered diagnostic of Addison disease.
Treatment
Often these animals present on emergency, so stabilizing the patient is always the first goal! Often this involves IV fluids to restore blood volume and correct any electrolyte abnormalities. Once a diagnosis of Addison disease is reached, the animal must be supplemented with exogenous glucocorticoids, such as dexamethasone or prednisolone, and exogenous mineralocorticoids, like desoxycorticosterone pivalate (DOCP).
For long term treatment, these animals most often need mineralocorticoid supplementation, with about 50% of animals also requiring additional glucocorticoid supplementation. It is important that owners are given supplemental glucocorticoids to give to their animals in times of stress or illness, as both of these situations may precipitate another Addisonian crisis.
Comparison Table
| Cortisol Dysfunction | Clinical Signs | Diagnostic Test | Treatment |
|---|---|---|---|
| Pituitary-dependent Hyperadrenocorticism | PU/PD Pot-belly Alopecia Hypercholesterolemia Elevated liver enzymes | LDDS for screening Possible HDDS | Mitotane Trilostane |
| Adrenal-dependent Hyperadrenocorticism | PU/PD Pot-belly Alopecia Hypercholesterolemia Elevated liver enzymes | LDDS for screening HDDS to confirm | Surgical removal of adrenal mass |
| Hypoadrenocorticism | Syncope Bradycardia Gastroenteritis | ACTH stimulation test | Supplementation with DOCP and glucocorticoids |
Check Your Understanding
Zachary JF. Pathologic Basis of Veterinary Disease, Sixth Edition.
Greco DS. Cushing Disease (Pituitary-dependent hyperadrenocorticism) in animals. Merck Veterinary Manual 2020.
Bruyette D. Addison Disease. Merck Veterinary Manual 2020.