The complement cascade is a series of proteins produced by the liver that are activated sequentially, and ultimately result in the formation of a pore in a target cell. Along the way, the different protein components can have other effects, like chemotaxis, opsonization and interaction with the immune system.
Table of Contents
Pathogenesis
Classical Pathway
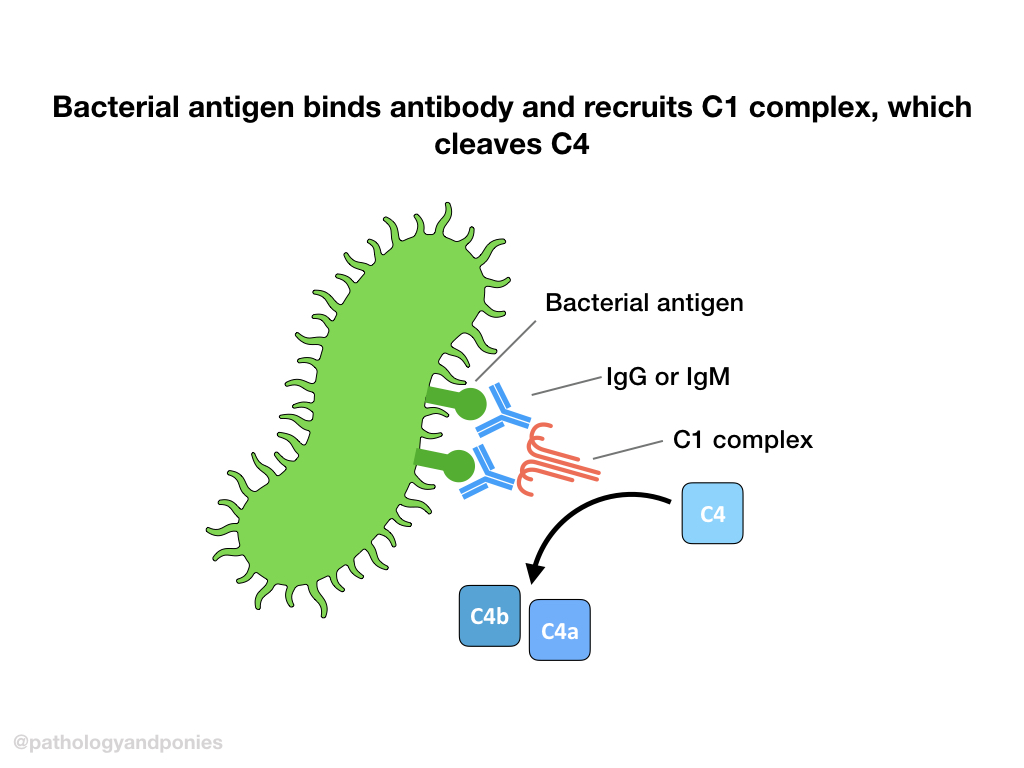
The classical pathway is activated by antibodies binding to an antigen and forming an antigen-antibody complex. C1 protein will cross-link bound IgG or IgM via the C1q region. This brings the C1r proteolytic component near the C1s component, which will be cleaved to form an active C1 complex.
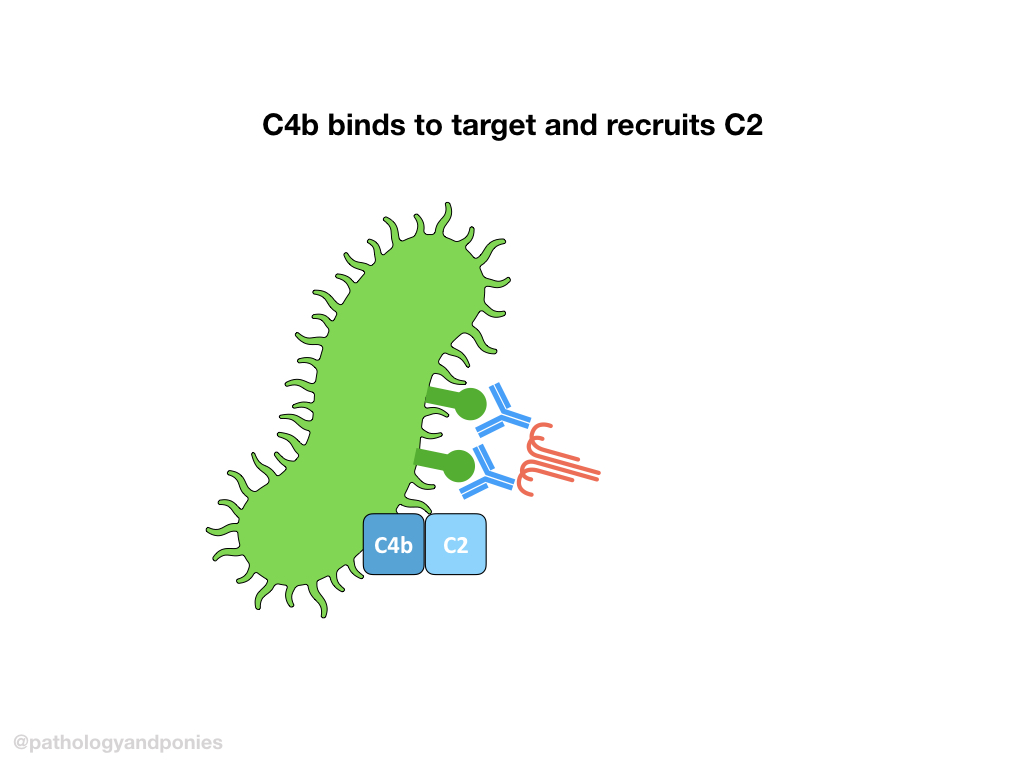
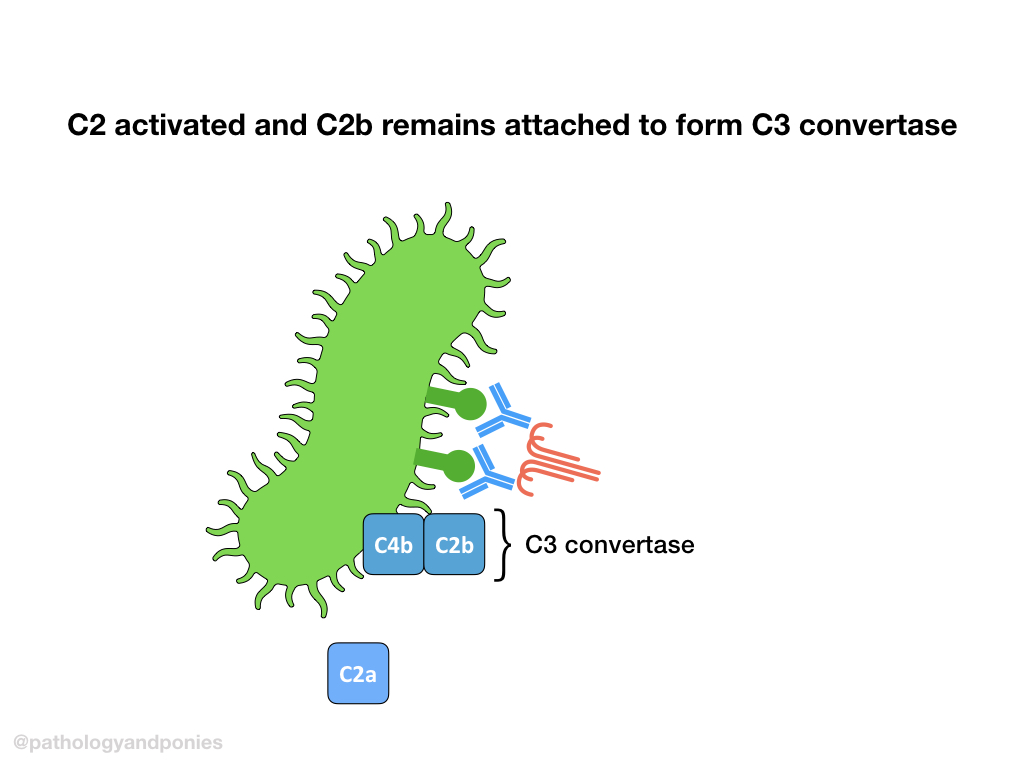
Activated C1 complex will cleave C4 into C4a and C4b. C4b will bind to the target cell and recruit C2, and cleave it into bound C2a and free C2b. This complex of C4b/C2a is called classical C3 convertase.
A brief note on classical C3 convertase:
Due to probably the most annoying nomenclature change ever, it is important to note that some newer sources refer to classical C3 convertase as C4b/C2b instead of C2a. This is because some sources now call the larger fragment of C2 C2b, whereas historically this fragment was called C2a. So… that’s cool I guess.
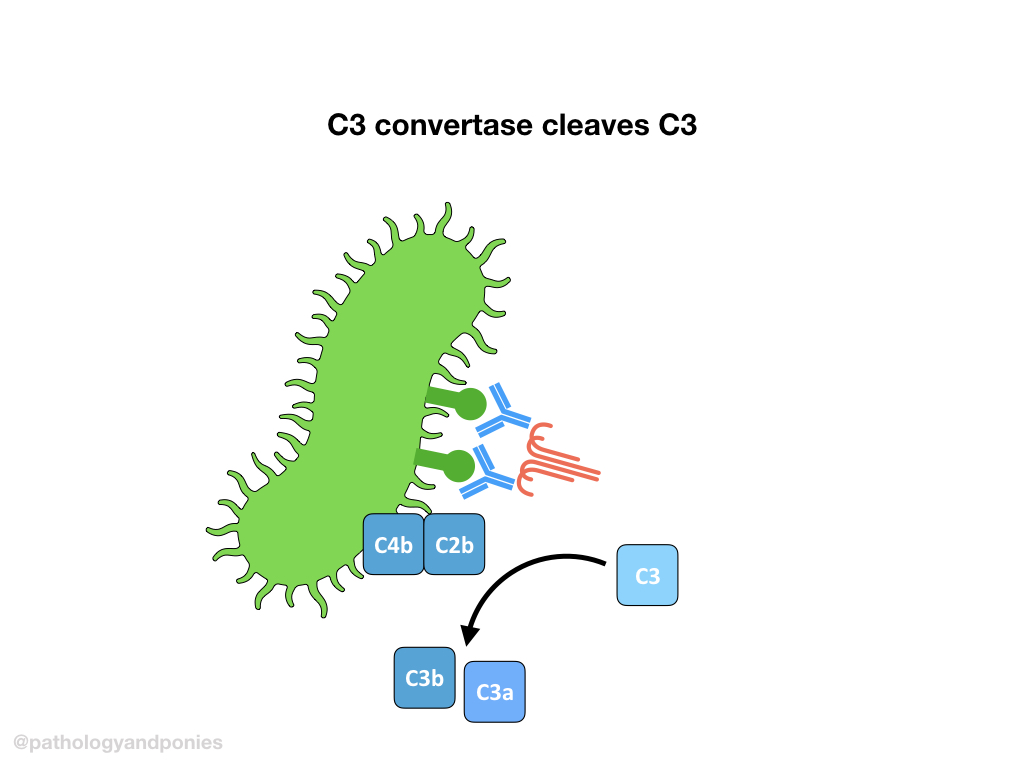
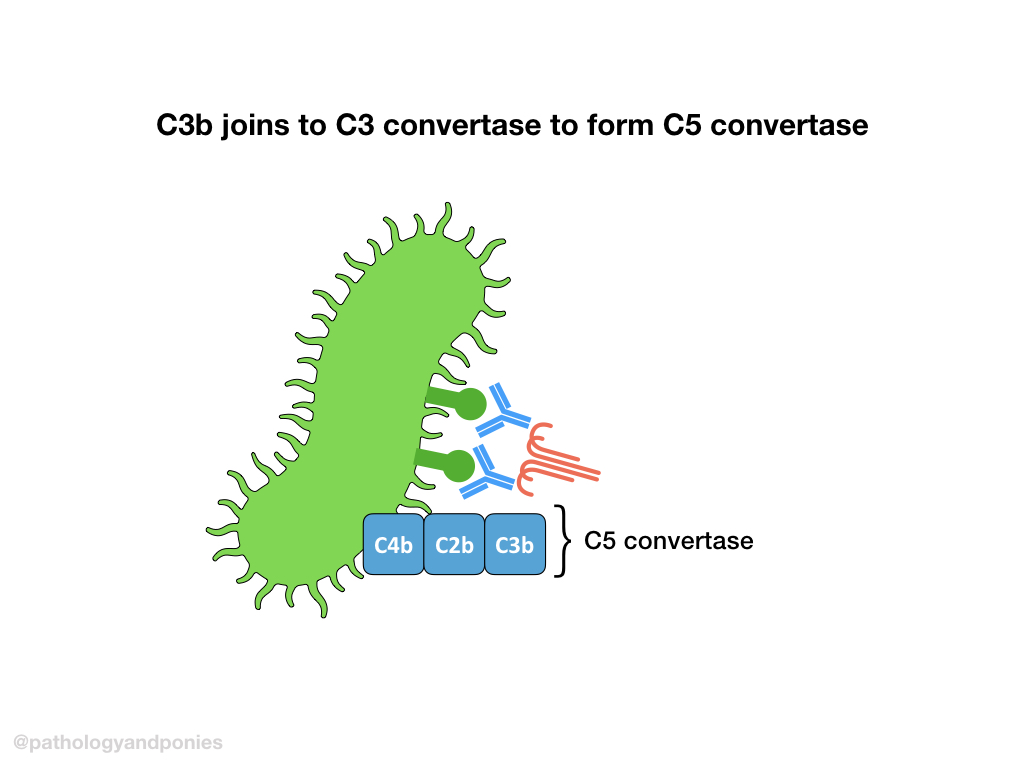
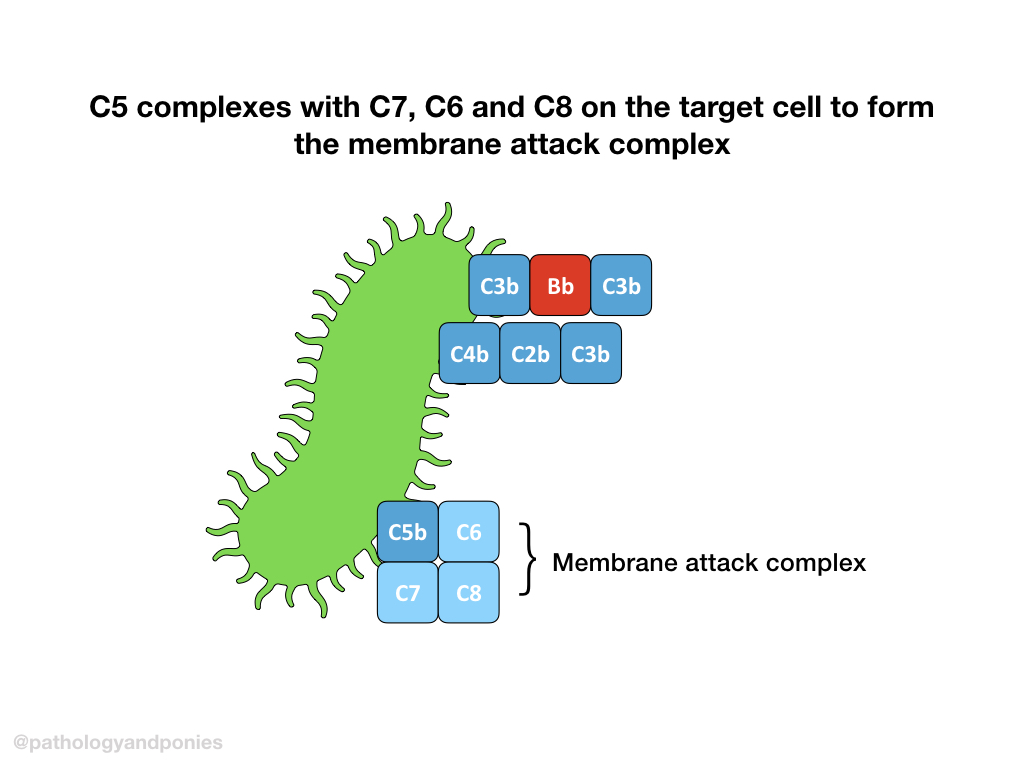
C3 convertase cleaves C3 into C3a and C3b. C3b then combines with classical C3 convertase (remember that’s C4b/C2a) to form classical C5 convertase (C4b/C2a/C3b).
Alternative Pathway
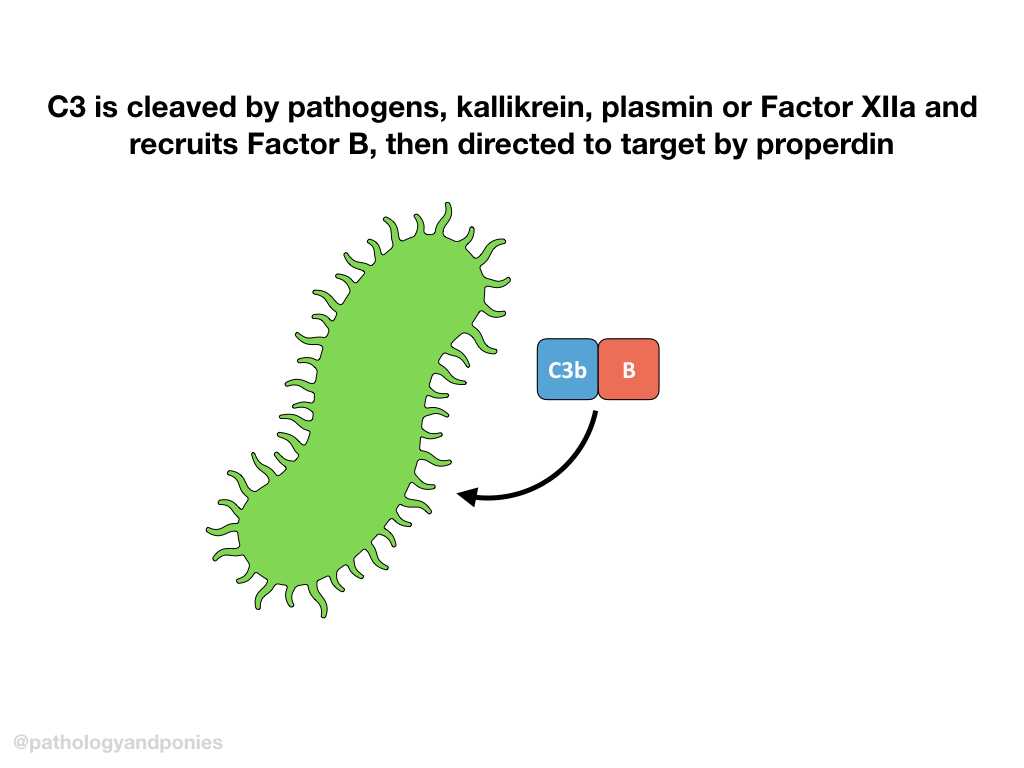
The alternative pathway is initiated by pathogen products, including LPS, fungal cell wall polysaccharides, etc. It can also be activated by kallikrein, plasmin and Factor XIIa, linking complement activation to clotting. These activating products all cleave C3 to form C3a and C3b.
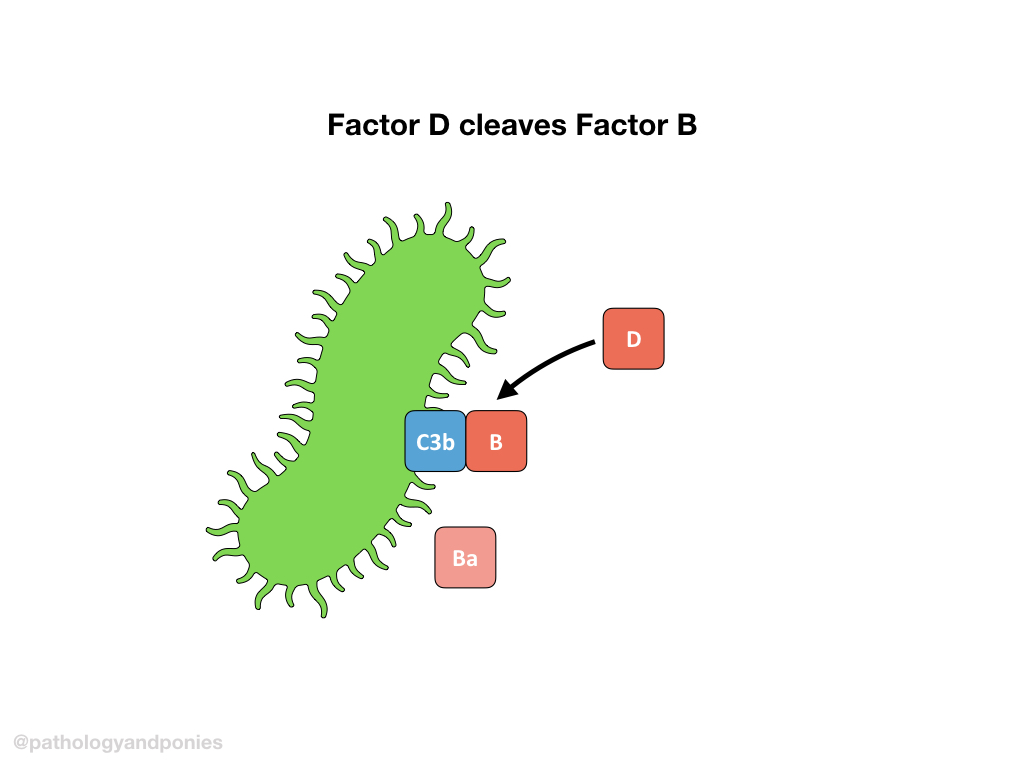
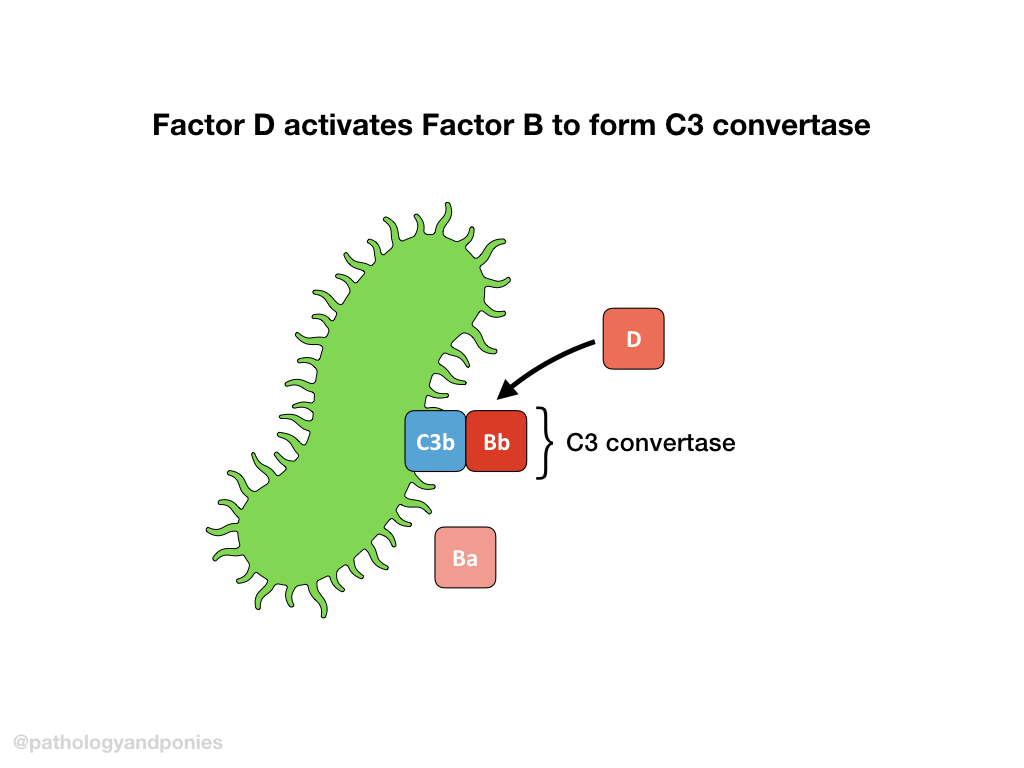
C3b forms a complex with Factor B. Properdin helps guide the C3b/Factor B complex to the target cell, and Factor D cleaves Factor B to form Ba and Bb. The complexed C3b/Bb is called the alternative C3 convertase.
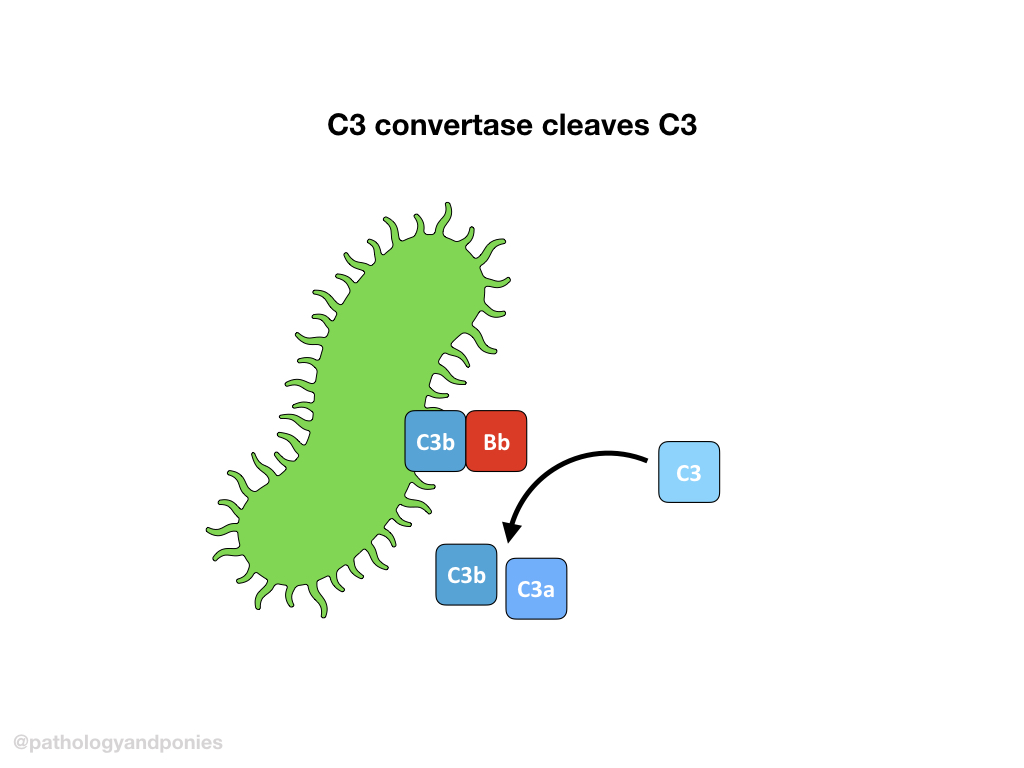
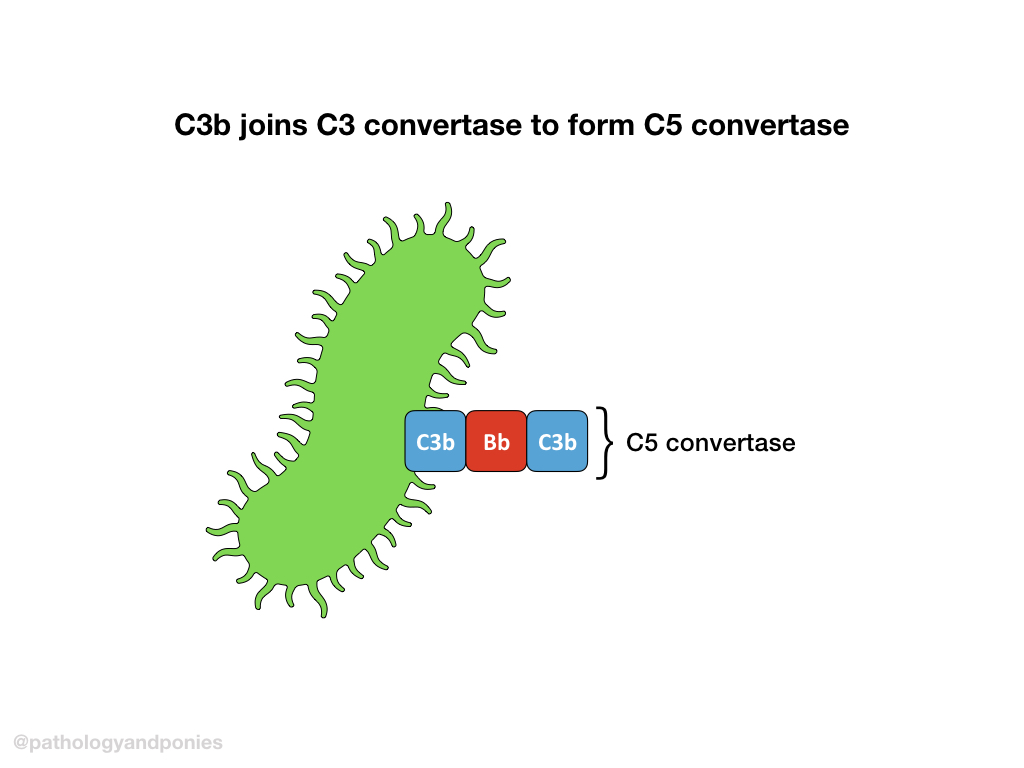
C3 convertase cleaves more C3 into C3a and C3b, and C3b complexes with the alternative C3 convertase to form the alternative C5 convertase (C3b/Bb/C3b).
Lectin Pathway
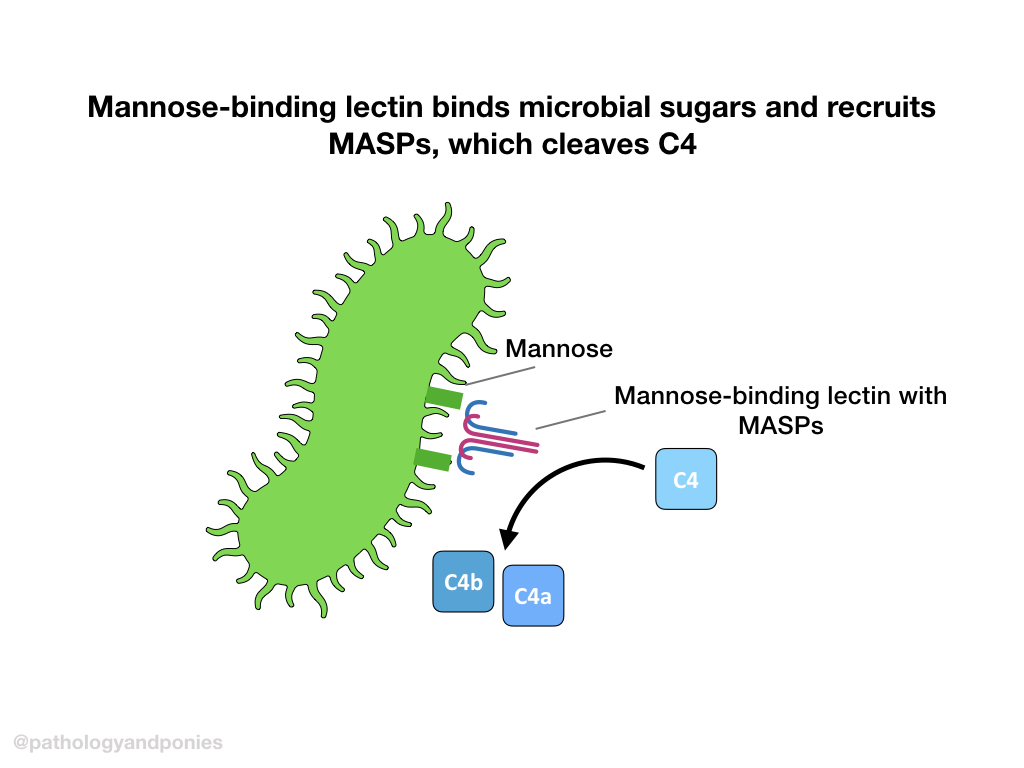
The final method of activation is induced by mannose-binding lectin binding to microbial sugars. These pattern recognition receptors recruit MASP 1 and 2 (MBL-associated serine proteases) to form the active MBL complex.
The active MBL complex cleaves C4 into C4a and C4b. C4b binds to the target cell, and enters the same route as the classical pathway.
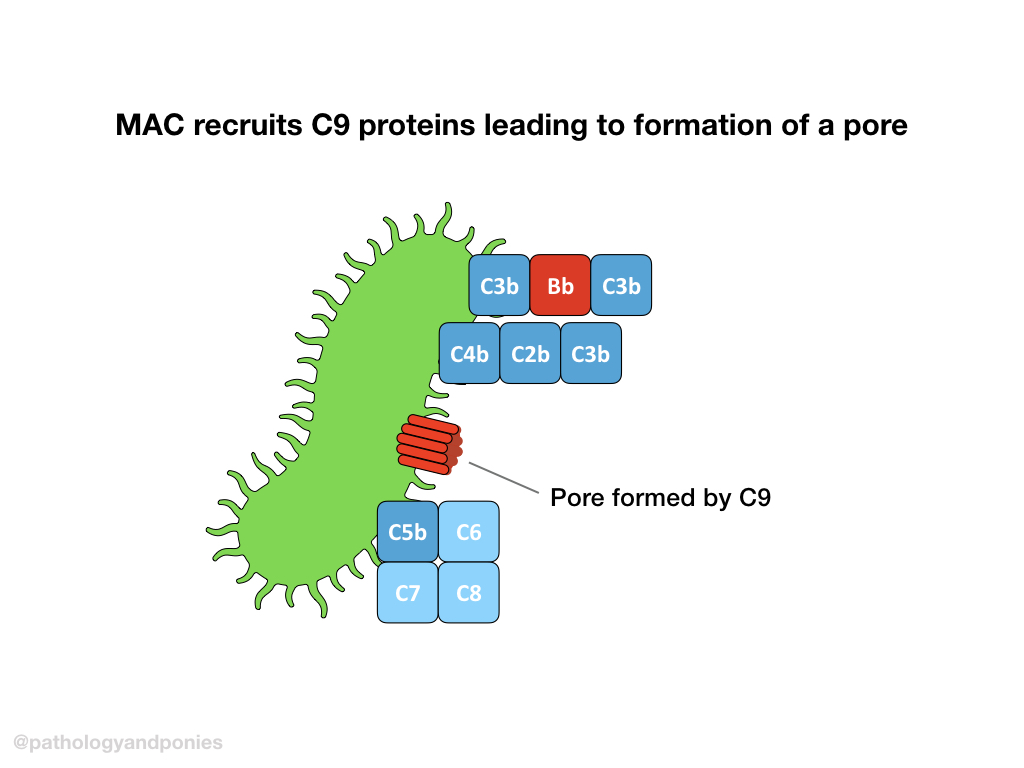
Terminal Pathway
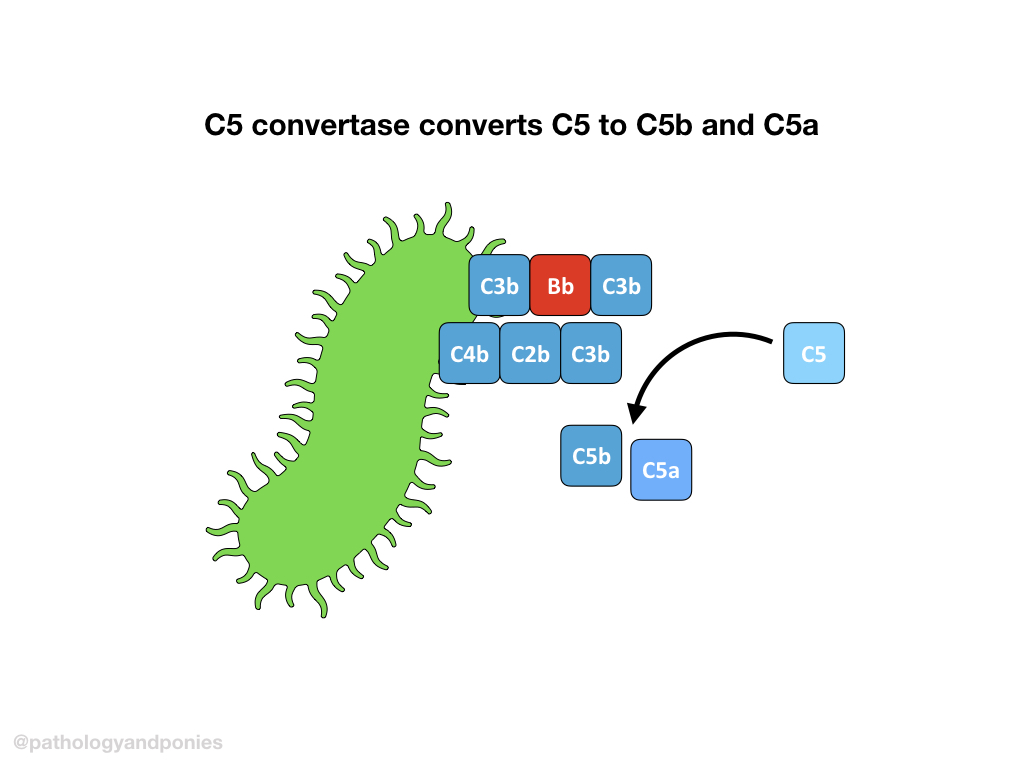
C5 convertase from any pathway cleaves C5 into C5a and C5b. C5b combines with C6, C7 and C8 to form the membrane attack complex. The MAC brings together multiple molecules of C9 to form a pore, completing the complement cascade.
Comparison Table
| Pathway | Stimulus | C3 Convertase | C5 Convertase |
|---|---|---|---|
| Classical | Antigen-antibody complexes | C4b/C2a | C4b/C2a/C3b |
| Alternative | Pathogen products Kallikrein Plasmin Factor XIIa | C3b/Bb | C3b/Bb/C3b |
| Lectin | Mannose-binding lectin | C4b/C2a | C4b/C2a/C3b |
Roles of Complement Products
As mentioned, the complement protein products can have other roles besides the complement cascade. These roles often serve as a link between the complement cascade and clotting, kinin and immunity.
| Activity | Complement Product | Mode of Action |
|---|---|---|
| Opsonization | Bound C3b and C4b | Phagocytes have CR1 receptor which binds C3b and C4b |
| Chemotaxis | Anaphylatoxins (C3a and C5a) | Leukocytes have anaphylatoxin receptors |
| Enhancing antibody response | Bound C3b and C4b | B cells and APCs have CR1 receptor which binds C3b and C4b |
| Enhancing T-cell response | Anaphylatoxins (C3a and C5a) | T cells and APCs have anaphylatoxin receptors |
| Reducing Treg function | Anaphylatoxins (C3a and C5a) | T cells and APCs have anaphylatoxin receptors |
| Clearing immune complexes | C1q with bound C3b/C4b Bound C3b and C4b | Phagocytes have CR1 receptor which binds C3b and C4b |
| Clearing apoptotic cells | C1q with bound C3b/C4b Bound C3b and C4b | Phagocytes have CR1 receptor which binds C3b and C4b |
Inhibition of Complement
There are several major regulators of complement that are important to know, particularly due to complement’s interconnectedness with other pathways.
| Inhibitor | Function | Location |
|---|---|---|
| C1 inhibitor (C1-INH) | Inactivates C1 complex Inactivates MBL complex | Plasma |
| Decay accelerating factor | Destabilizes C3/C5 convertases | Membrane-bound |
| Factor H | Binds C3b Blocks formation of C3 convertase | Plasma |
| Thrombomodulin | Enhances Factor H activity Activates thrombin-activatable fibrinolysis inhibitor (TAFI) to inactivate anaphylatoxins | Membrane-bound |
| CD59 | Blocks C9 from associating with MAC | Membrane-bound |
Disorders
In humans, there have been several mutations affecting complement factors that have been identified. The most common deficiency is C2 deficiency, although it appears that these patients have little to no increase in infection risk. This suggests that the lectin and alternative pathways are adequate to prevent infection. The most important complement deficiencies in our animal species affect C3 and Factor H.
C3 Deficiency
Because all of the pathways require C3 cleavage, this deficiency has serious consequences. This deficiency has been previously described as an autosomal recessive condition in Brittany spaniels, which causes a premature stop codon in C3 translation. These dogs are at an increased risk of recurrent infections including pneumonia, septicemia and pyometra.
Factor H Deficiency
Factor H deficiency has been described in Norwegian Yorkshire pigs, and is linked to genetic mutations that prevent secretion of Factor H from hepatocytes. Because Factor H blocks C3 convertase formation, deficiency of this protein results in uncontrolled formation of C3 convertase and cleavage of large amounts of C3. This results in glomerulonephritis due to glomerular deposition of C3 components.
C1 Inhibitor Deficiency
Although C1-INH deficiency has not been described in animals, it is an important autosomal dominant disease in humans, causing hereditary angioedema. In this condition, the lack of C1-INH results in large amounts of bradykinin production, leading to episodes of edema in the skin, airways and gastrointestinal tract.
Zachary JF. Pathologic Basis of Veterinary Disease, Sixth Edition.
Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease, Tenth Edition.