Cellular signalling can lead to differentiation, proliferation or other functions. There are many different types of cell signals, involving different signalling pathways and transcription factors, which the cell integrates to determine what it should do next.
Types of Signals
Cell signals can fall into a few major types:
- DAMPs and PAMPs. Damage-associated molecular patterns and pathogen-associated molecular patterns allow cells to respond to cellular damage or infections.
- Cell-cell contact. Cell connections are mediated by adhesion molecules or gap junctions made of connexon channels. These cellular connections can stimulate signalling consistently, and when disrupted cells may activate different signalling pathways.
- Cell-ECM contact. Similar to cell to cell contact, cells also need contact with the extracellular matrix to activate signalling pathways. This cell-ECM contact is mediated by integrins.
- Secreted molecules. This category includes things like growth factors, cytokines and hormones.
These cell signalling molecules bind to specific receptors in or on the cell. Ligands that are lipid-soluble are often able to diffuse through the plasma membrane to reach intracellular receptors, whereas lipid-insoluble ligands bind to cell-surface receptors. Lipid-soluble ligands can act on the nucleus directly after binding to a receptor protein, or activate cytosolic signalling pathways. Cell-surface receptors can either open an ion channel, activate a G protein, activate an enzyme like tyrosine kinase, or trigger a change in protein binding or stability to activate a transcription factor.
Signal Transduction Pathways
Once a ligand binds, a signalling transduction pathway is activated. There are several different types of signalling pathways with various proteins involved.
Receptors with Kinase Activity
Kinase activity results in phosphorylation of downstream target molecules. There are three major types:
- Tyrosine kinases, which phosphorylate tyrosines.
- Serine/threonine kinases, which phosphorylate serines or threonines.
- Lipid kinases, which phosphorylate lipids.
Each phosphorylation event also includes the activity of a phosphatase, which “undoes” the phosphorylation and allows for control of the signalling pathway. These receptors can either have their own intrinsic kinase activity (receptor tyrosine kinases), or can recruit tyrosine kinases from the cytosol (nonreceptor tyrosine kinases). In the case of receptor tyrosine kinases, ligand binding induces cross-linking between multiple receptors, activating the tyrosine kinase domains in their cytoplasmic tail. Nonreceptor tyrosine kinases bind to the receptor after ligand binding, and phosphorylate the receptor or other proteins.
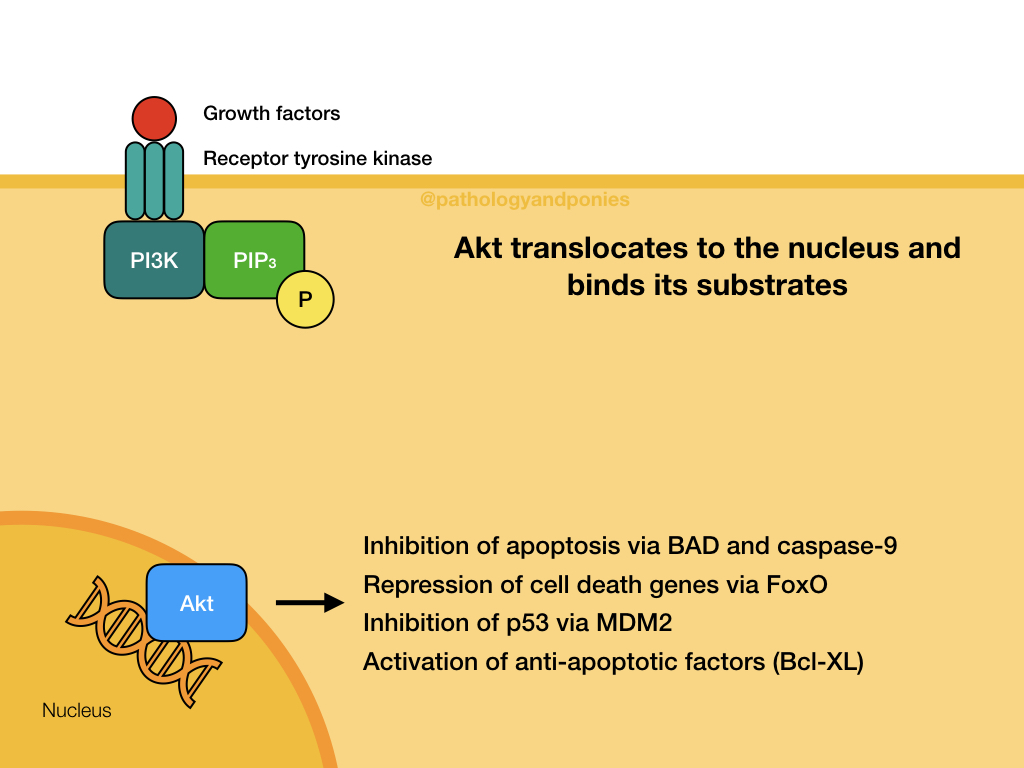
Akt/PKB Pathway
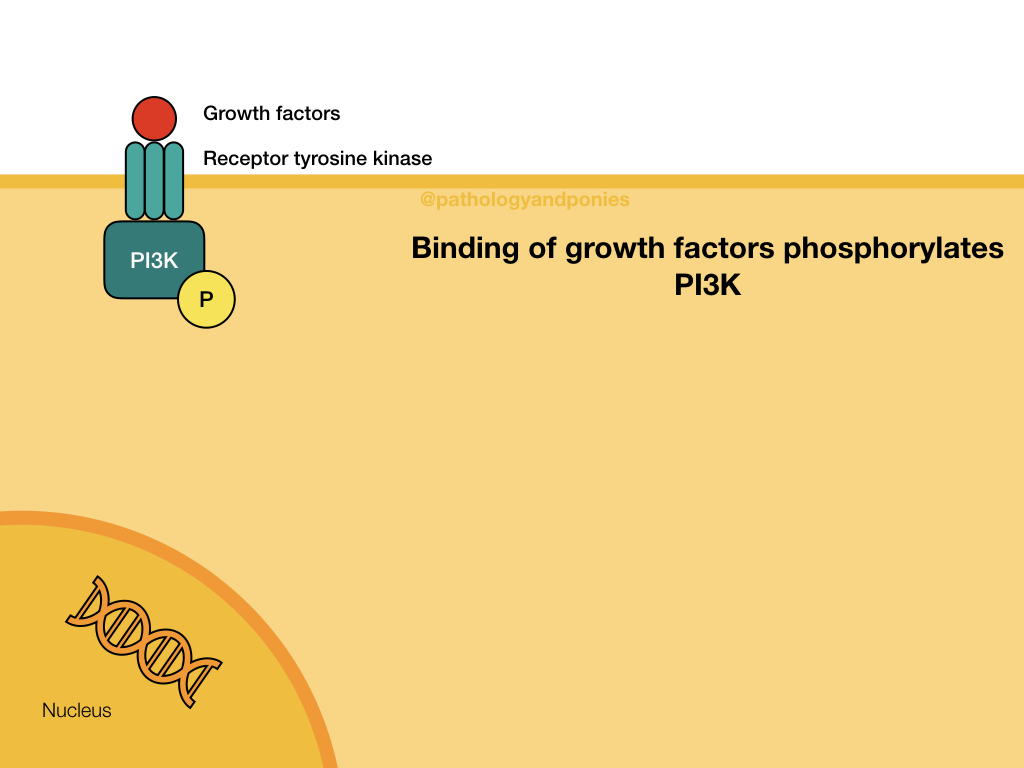
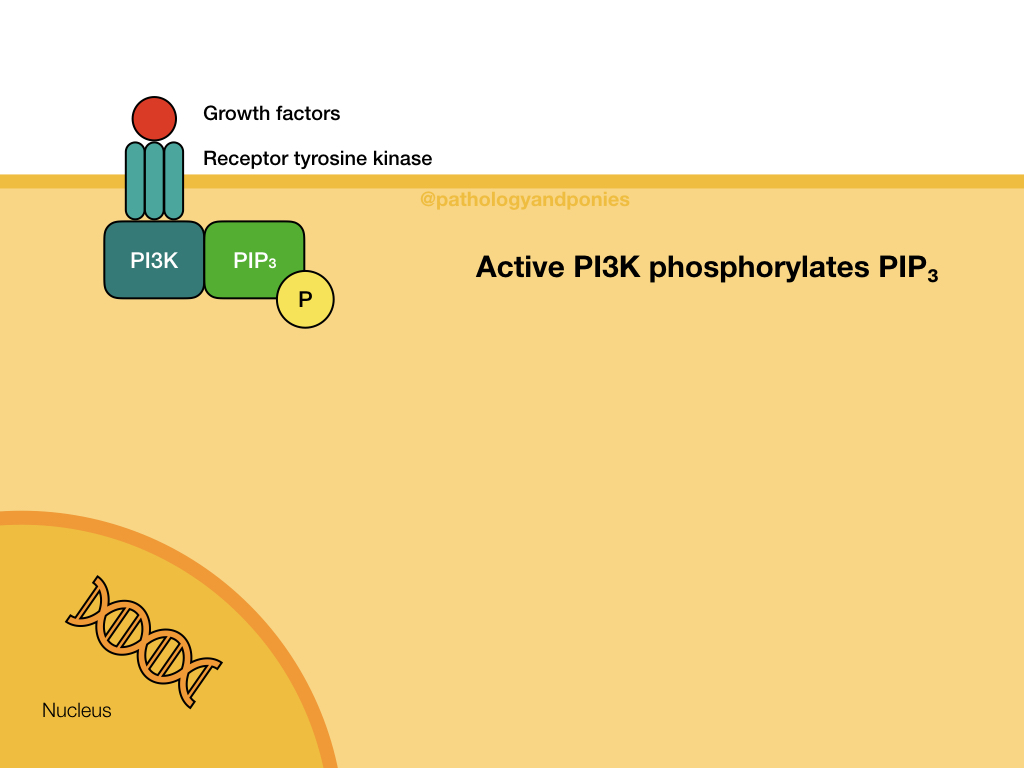
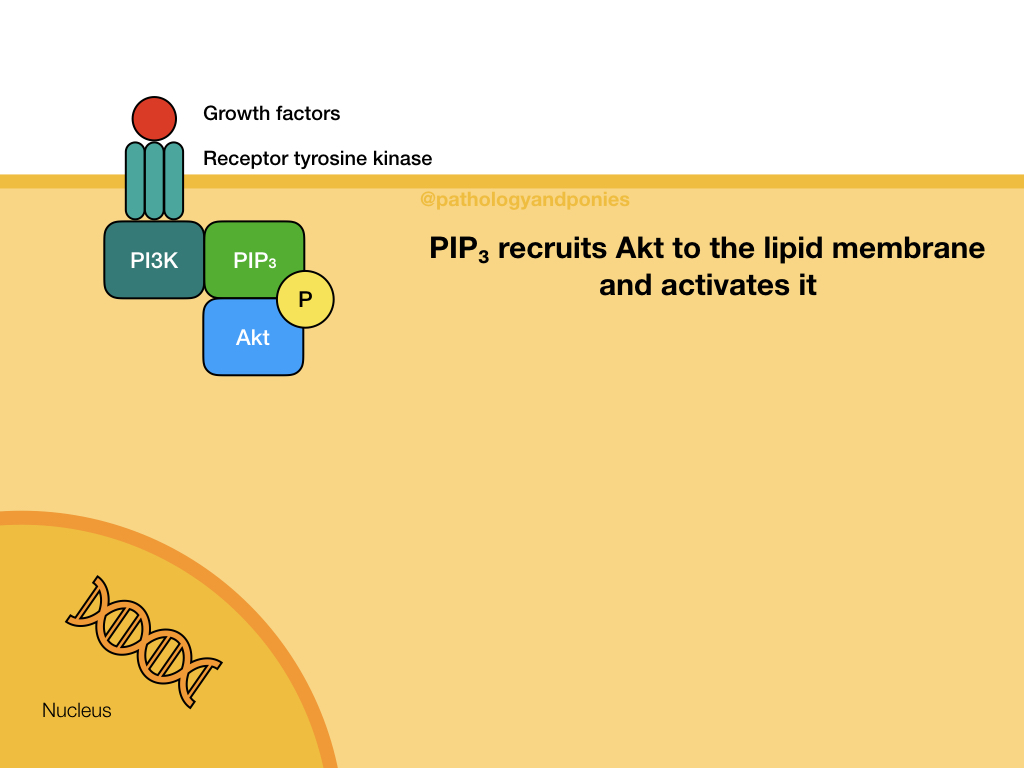
The Akt/PKB pathway is also known as the PI3K/Akt pathway, due to the two major proteins involved. This pathway is activated by growth factor binding to a receptor tyrosine kinase or a G-protein coupled receptor (described later). This activates the surface receptor and leads to phosphorylation of PI3K. Activated PI3K phosphorylates lipids in the cell membrane to form PIP3, which acts as a secondary messenger. PIP3 recruits the serine/threonine kinase Akt to the cell membrane, where it becomes activated. Activated Akt translocates to the nucleus to bind to its substrates.
Akt has numerous effects, with the end goal of promoting cell survival and growth. Some notable effects:
- Phosphorylation of FoxO transcription factors, which marks them for degradation. These leads to repression of cell death signalling by these factors. Notably, Akt phosphorylates FoxO4, which prevents expression of p27.
- Translocation of NFκB to the nucleus, which promotes expression of Bcl-XL which inhibits apoptosis.
- Phosphorylation of MDM2, to increase its ubiquitination of p53 and prevent apoptosis.
- Phosphorylation of BAD, a pro-apoptotic regulatory protein.
- Phosphorylation of caspase-9, to prevent the mitochondrial pathway of apoptosis.
- Preventing degradation and promoting translation of cyclin D.
- Increasing degradation of p21.
- Activation of mTOR, a central regulator of metabolism. mTOR has several important outcomes if upregulated in tumours:
- Increases protein synthesis, allowing for increased cell cycle progression.
- Inhibits autophagy.
- Increases angiogenesis by translation of HIF1A.
- Upregulation of PKM2 which drives the Warburg effect.
This pathway is primarily regulated by phosphatase and tensin homolog (PTEN), which converts PIP3 into inactive PIP2. Loss of PTEN activity is a common finding in cancers.
An important activator of this pathway is insulin. In this role, Akt activates GLUT4, which promotes transportation of glucose into the cell.
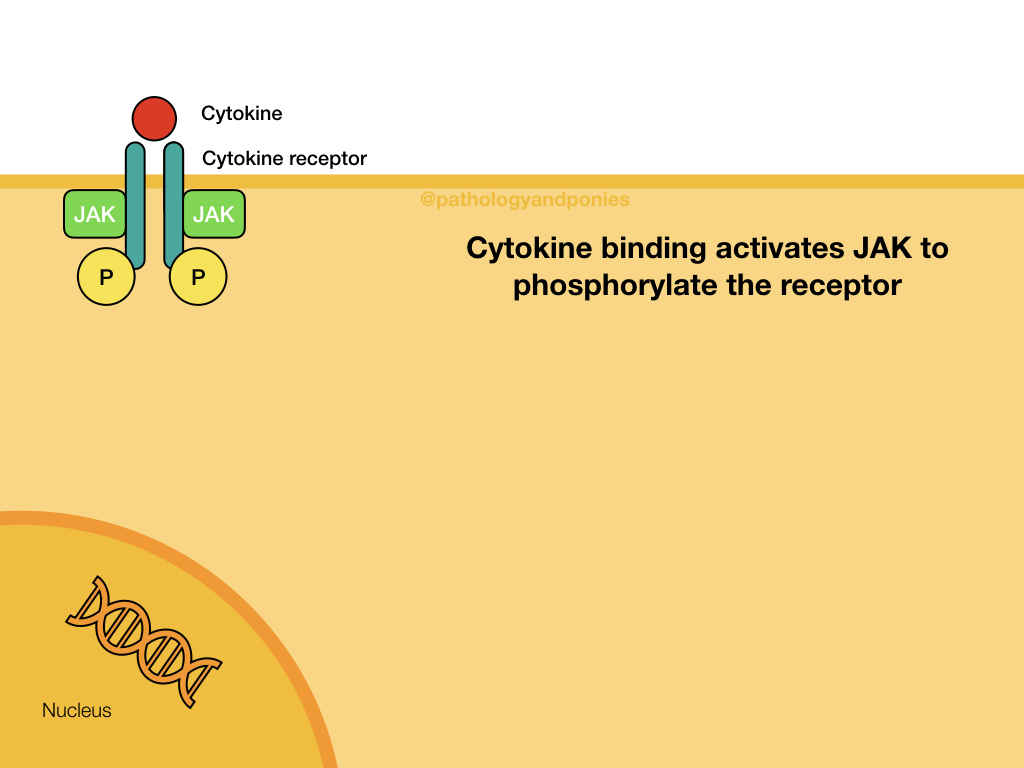
JAK/STAT Pathway
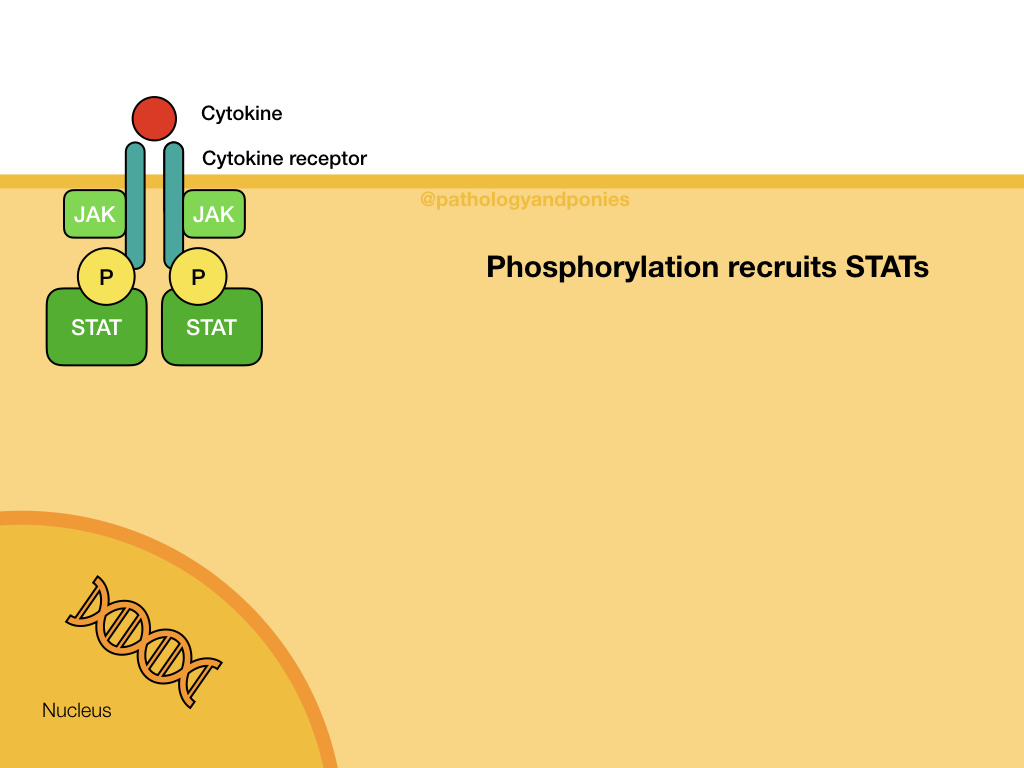
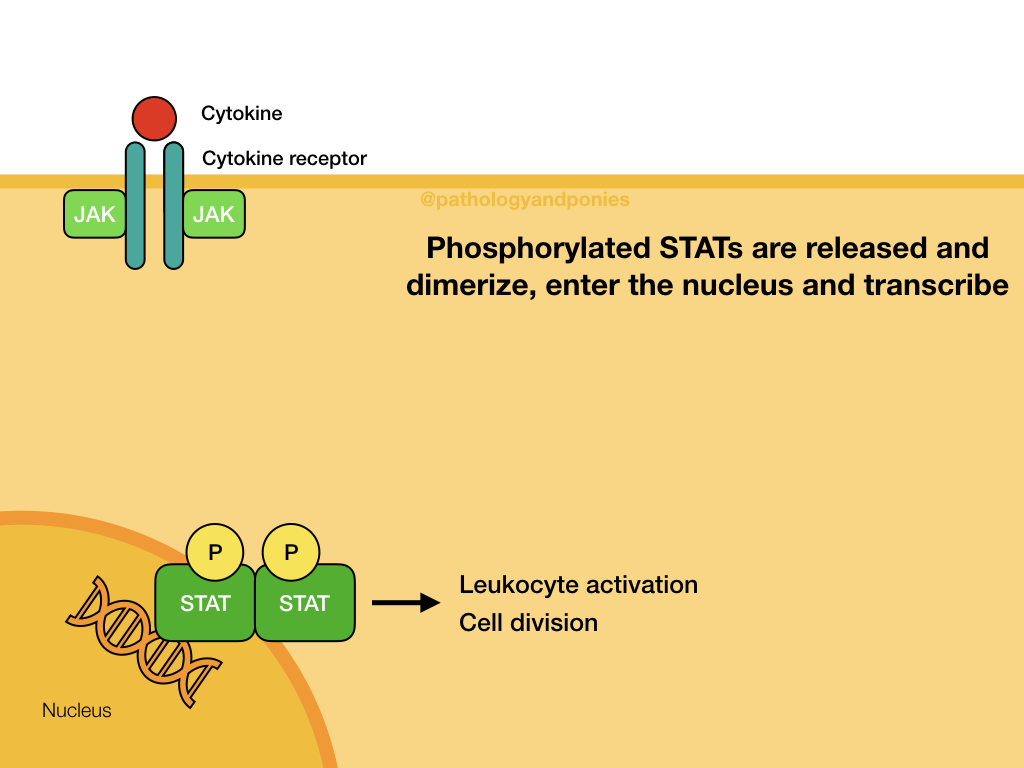
JAK (Janus kinases) are a common finding attached to the intracellular domain of cytokine receptors. These kinases have tyrosine kinase activity, giving the cytokine receptor a receptor tyrosine kinase function. When a cytokine, such as an interleukin or interferon, binds to the cytokine receptor, the activated JAKs phosphorylate the receptor to activate it. The receptor then binds STATs, which become phosphorylated and detach from the receptor. The STATs form a dimer that translocates to the nucleus and activates target genes.
The JAK/STAT signalling pathway is primarily used for cytokine receptor signalling, leading to gene transcription that promotes immune cell proliferation, activation and recruitment. Some example roles in immunity:
- Leukocyte development in response to IL-2, IL-4, IL-10 and IFN-ɣ.
- Activation of NK cells.
- Stimulation of B-cell proliferation and production of antibody.
JAK/STAT is also involved in cell division, particularly of leukocytes. For example, increased STAT3 activates transcription of BCL2 and Myc, promoting cell division. Excessive JAK/STAT stimulation can lead to cancer development, and generally is associated with more malignant tumours. As might be expected, its role in leukocyte cell division makes this tumour a common site of mutation in leukemia and lymphoma.
JAK/STAT is regulated by protein inhibitors of activated STAT (PIAS), protein tyrosine phosphatases (PTPs) and suppressors of cytokine signalling (SOCS).
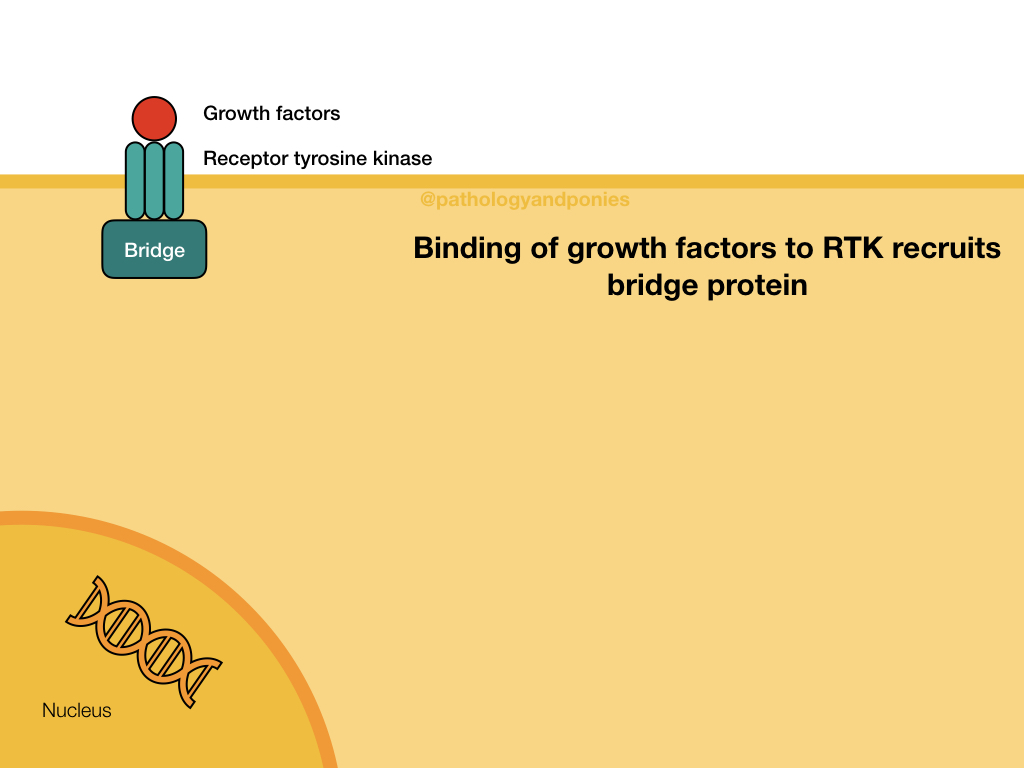
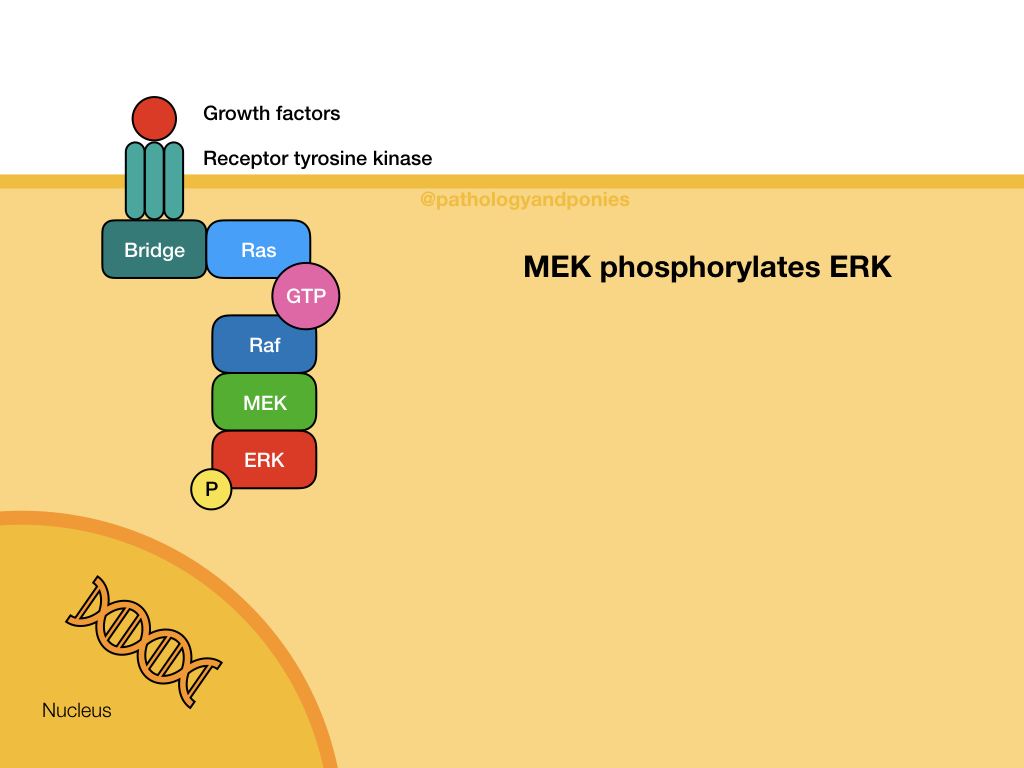
Ras/Raf/MEK/ERK Pathway
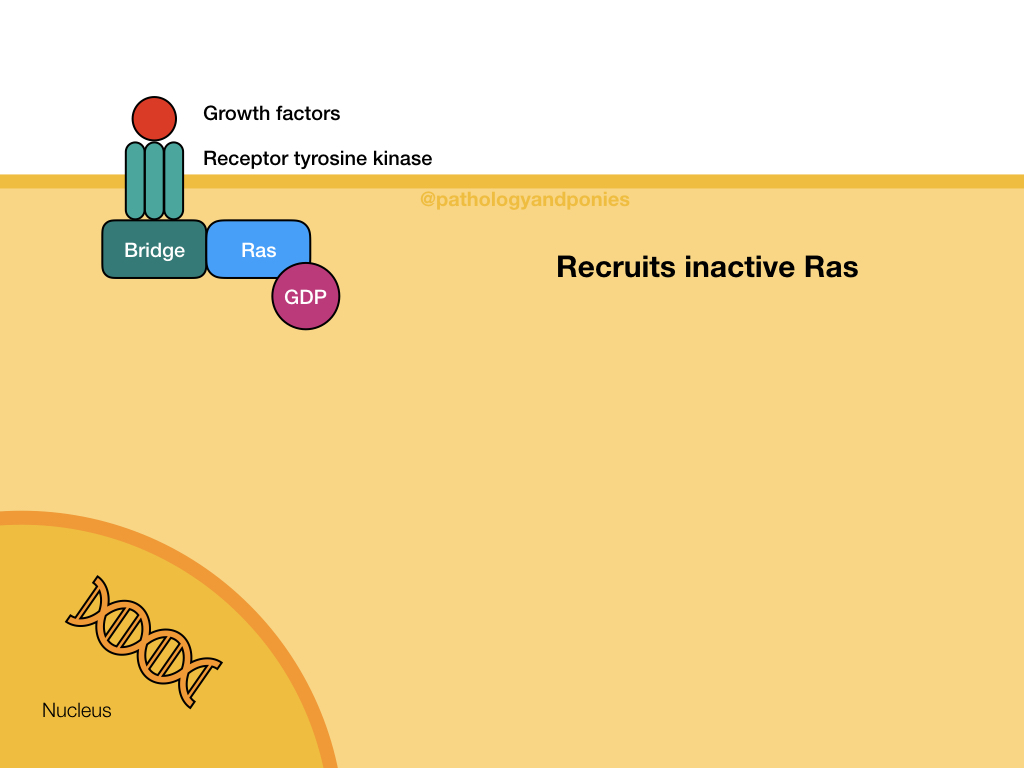
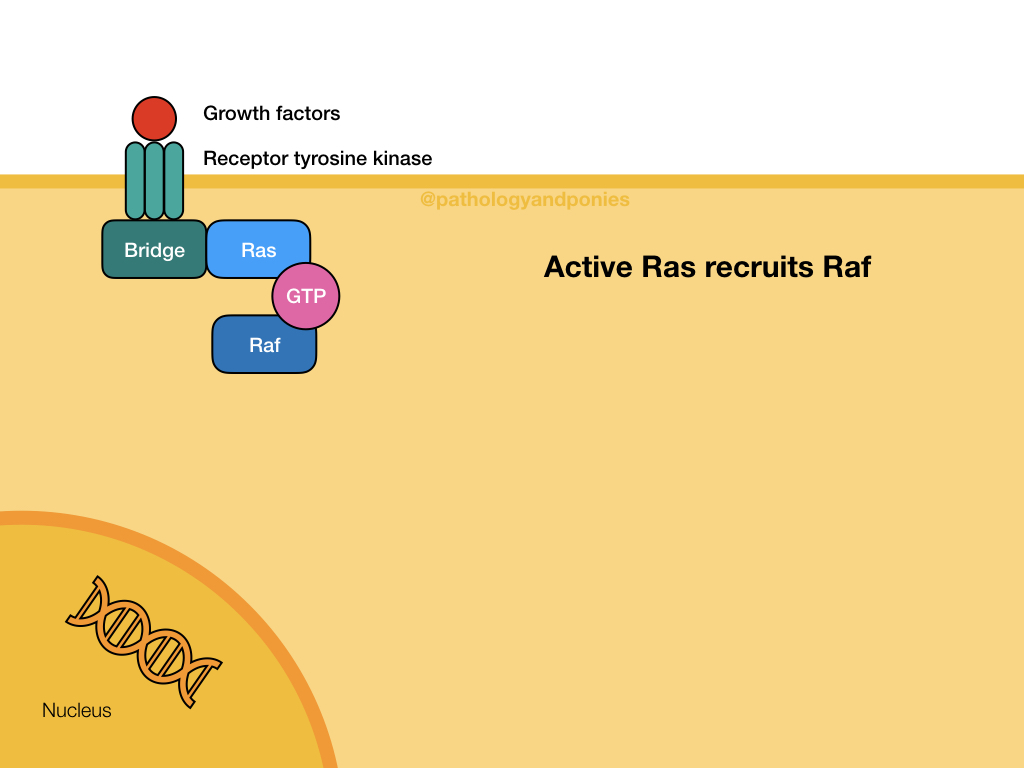
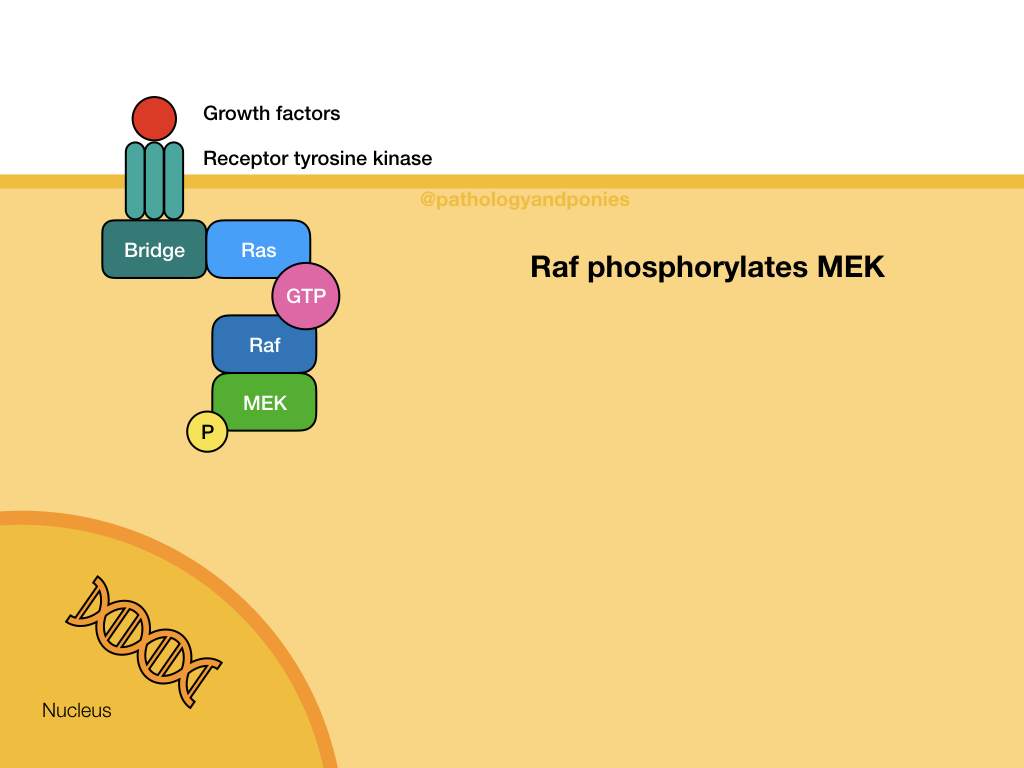
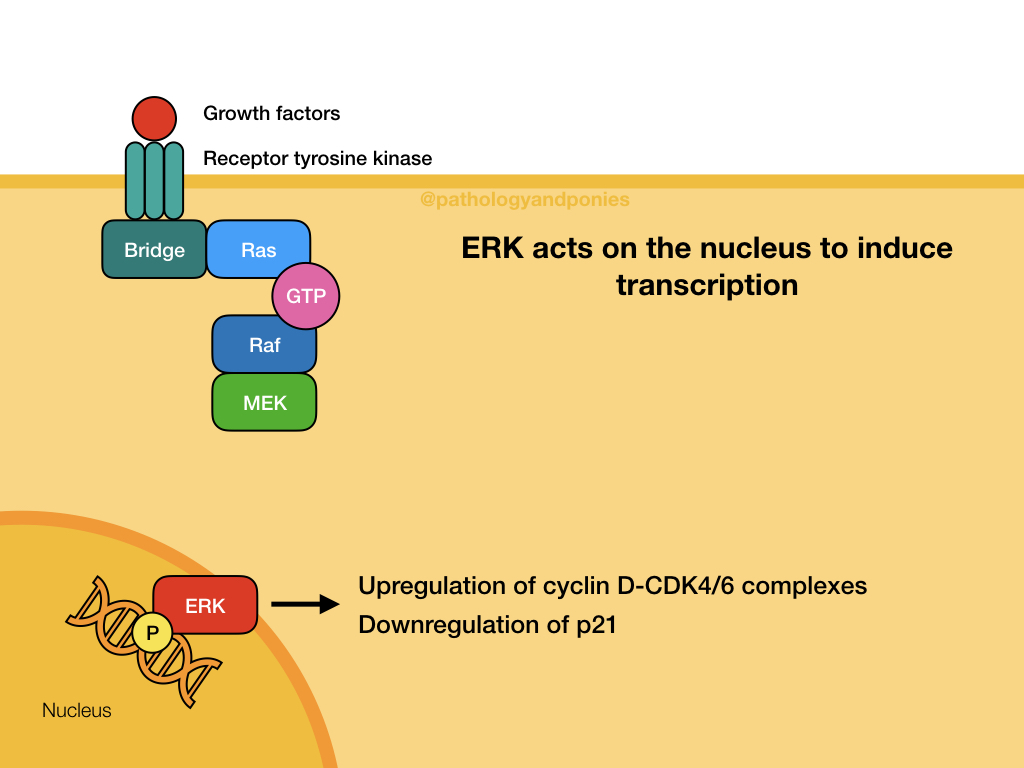
This pathway is a common signalling pathway for mitogens, which induce cell division. For example, growth factors like epidermal growth factor, platelet-derived growth factor and fibroblast growth factor. In this pathway, the mitogen binds to a cell surface receptor with tyrosine kinase activity. The receptor becomes phosphorylated and recruits a bridge protein. The bridge protein binds to inactive Ras, which has a GDP. Binding to the bridge protein converts the GDP to a GTP, activating Ras. Ras activates Raf, which phosphorylates MEK. MEK phosphorylates ERK, which translocates to the nucleus to induce transcription. ERKs are also known as mitogen-activated protein kinases (MAPKs).
MAPK or ERK activation leads to activation of Myc, which upregulates cyclin D-CDK4/6 complexes and downregulates p21 to promote cell proliferation. Uncontrolled MAPK signalling is a common defect in cancers, due to Myc’s important role in cell proliferation.
Activation of MAPK in a normal cell is regulated by the intrinsic GTPase activity of Ras, which converts the GTP of its active form back into GDP, inactivating the protein.
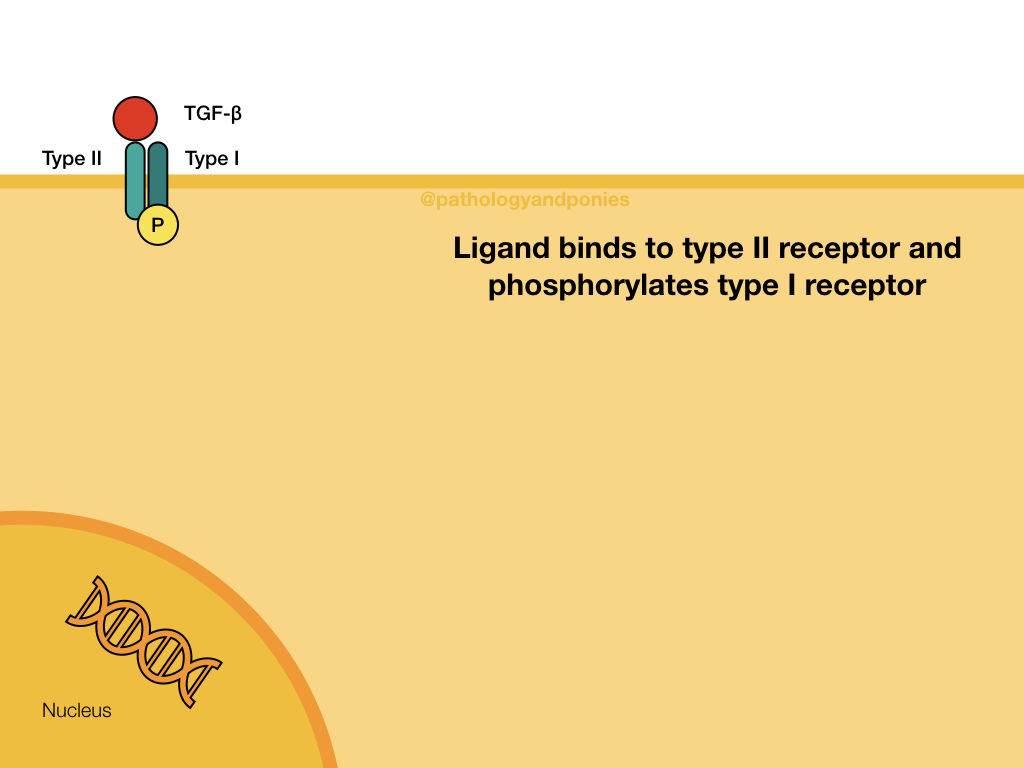
TGF-beta Pathway
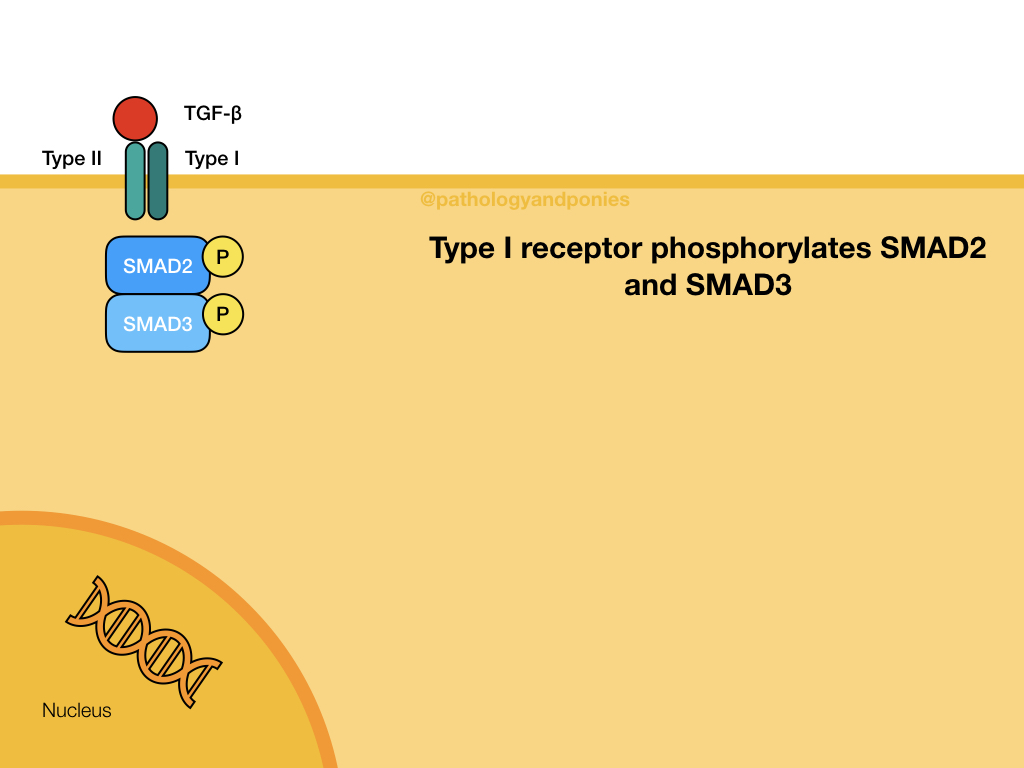
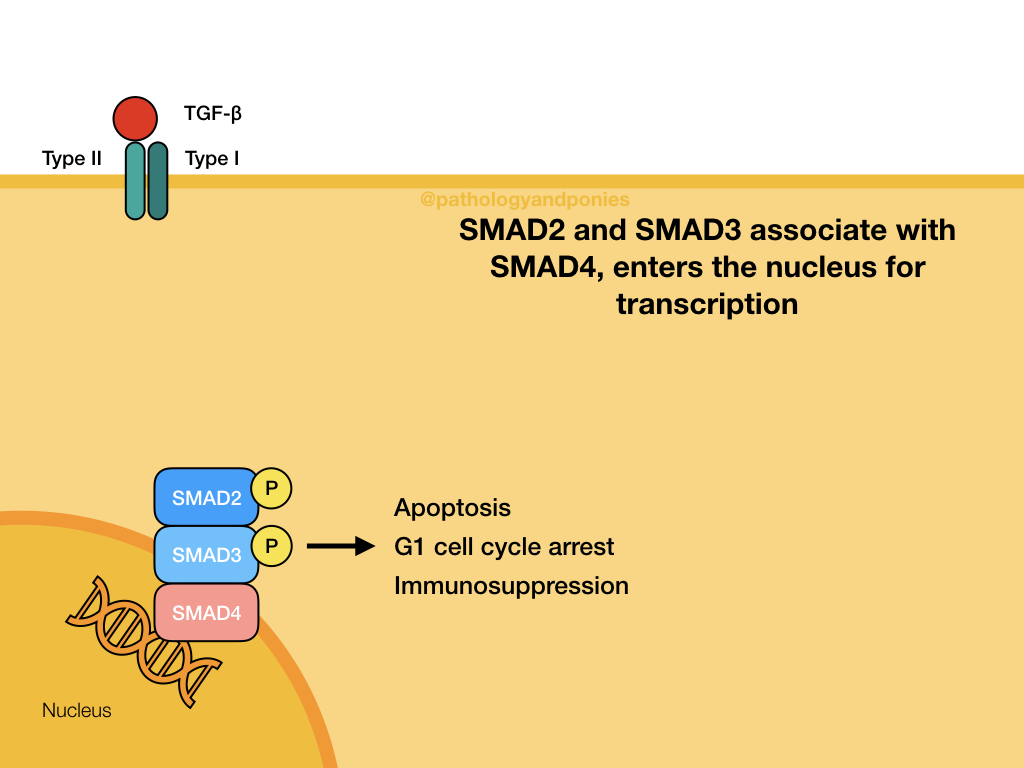
TGF-beta binds to a TGF beta type II receptor, which has serine/threonine kinase activity. The type II receptor phosphorylates the type I receptor. The type I receptor phosphorylates the two R-SMADs, SMAD2 and SMAD3. These phosphorylated SMADs have a high affinity for SMAD4, which is the main coSMAD. They form a complex which enters the nucleus to transcribe genes.
TGF-beta binding typically transcribes genes involved in apoptosis, G1 cell cycle arrest and immunosuppression.
The cycle is regulated by inhibitory SMADs SMAD6 and SMAD7. SMAD7 prevents phosphorylation of the R-SMADs. SMAD6 binds SMAD4 and prevents it from acting as a coSMAD. SMURF proteins can also regulate the pathway, by ubiquitinating SMADs to target them for proteosomal degradation.
G-protein Coupled Receptors
The characteristic shape of GPCRs is a protein that traverses the cell membrane seven times. When a ligand binds, GPCR associated with G protein, an intracellular GTP-binding protein. G protein contains a GDP, which becomes activated to GTP after binding to the GPCR. The GPCR then mediates other signalling pathways.
cAMP-dependent Pathway
This pathway is also known as the adenylyl cyclase pathway, and is initiated by ligand binding to a GPCR. After activation of the GPCR, the receptor activates adenylyl cyclase, which converts ATP into cyclic adenosine monophosphase (cAMP). cAMP acts as a secondary messenger that activates protein kinase A. Protein kinase A has numerous effects depending on the cell it resides in, but its basic function is regulation of glucose and lipid metabolism.
The cAMP pathway is regulated primarily by G-protein-mediated hydrolysis of bound GTP back to GDP, allowing for receptor de-activation. It can also be regulated by cAMP phosphodiesterase which converts cAMP to AMP.
Phospholipase C Pathway
The phospholipase C pathway is activated by GPCRs, such as serotonin, histamine and adrenergic receptors. Binding of the ligand to the receptor activates phospholipase C, which cleaves PIP2 into DAG and IP3. DAG remains bound to the cell membrane, and IP3 is released into the cytoplasm. IP3 binds to receptors on calcium channels in the smooth endoplasmic reticulum, causing calcium concentrations within the cytoplasm to increase. The increased calcium combines with DAG, resulting in activation of protein kinase C. IP3 also activates protein kinases and transcription.
Phospholipase C is also critical in the release of arachidonic acid from phosphatidylinositol and phosphatidylcholine. This allows for production of lipid mediators.
Frizzled Receptors
Frizzled receptors are part of the Wnt pathway, and are a type of GPCR. This pathway controls beta-catenin levels within the cell. Wnt protein binds to the Frizzled receptor and sends a signal to Dishevelled protein in the cytoplasm. Dishevelled disrupts the destruction complex for beta-catenin which is composed of APC and Axin. Without Wnt binding, this complex normally ubiquitinates beta-catenin for destruction. In the absence of the destruction complex, beta-catenin translocates to the nucleus and activate TCF transcription factors. These transcription factors activate genes that promote cell survival and cell proliferation, such as cyclins, Myc and JUN.
Patched Receptors
Patched are a unique receptor type that are exclusively part of the Hedgehog pathway.
Sonic Hedgehog Pathway
Sonic hedgehog is a protein involved in cell differentiation and proliferation. In its pathway, it binds to Patched-1 receptor. At rest, Patched-1 receptor inhibits Smoothened protein, so after binding Smoothened begins to accumulate within the cell. Smoothened activates GLI transcription factors which accumulate in the nucleus and mediate the effects of Sonic hedgehog.
In developing animals, Sonic hedgehog is involved in development of digits on limbs. In adults, it promotes proliferation of stem cells such as hematopoietic cells, and also has a role in hair follicle growth. Notably in tumours, Sonic hedgehog activation increases angiopoietins, leading to angiogenesis, cyclins, promoting cell growth, and anti-apoptotic genes.
Notch Receptors
Notch is a fairly simple pathway, but is involved in several different functions. The main ligand for this pathway is transmembrane proteins on a neighbouring cell. Thus, Notch is mainly triggered by direct cell-to-cell contact. When a ligand binds to Notch, the receptor becomes proteolytically cleaved to release a fragment (intracellular Notch), which forms a transcription complex.
Notch’s major function is in angiogenesis, where it promotes budding of new vessels at carefully regulated intervals. Delta-like ligand 4 (DII4) is a Notch ligand expressed at the tip of endothelial cells. This ligand expression activates Notch in adjacent cells, and prevents their migration and development, allowing a single vessel to form in the face of high VEGF signalling. Notch also helps regulate embryogenesis, particularly int he brain, cardiovascular system and endocrine tissue.
Toll-Like Receptors
The primary role of TLRs is being pattern recognition receptors, to recognize pathogens and activate cells appropriately. These receptors are found on leukocytes, epithelial cells, endothelial cells and fibroblasts primarily. Different TLRs recognize different aspects of pathogens, which are summarized here.
After activation, TLRs recruit an adaptor protein (AP-1) in the cytosol to activate further signalling. There are four main adaptor proteins: MyD88, TRIF, TIRAP and TRAM. MyD88 and TRIF are the most important, and have their own pathway.
The MyD88-dependent pathway is used by every TLR, except TLR3. MyD88 is recruited to the TLR and activates various kinases. The pathway results in activation of NFκB and MAPK (mitogen-activated protein kinases).
The TRIF-dependent pathway is used by TLR3 and TLR4, after binding of dsRNA and LPS respectively. The TLR receptor then recruits TRIF, which activates kinases that ultimately phosphorylate IRF3 (interferon regulatory factor). IRF3 crosses into the nucleus and produces interferon type I.
TLR4 is the only TLR that recruits all four adaptor proteins, and thus has the widest array of effects. TLR4 bound to LPS binds to MD2 to form a complex, which recruits TIRAP and MyD88. The complex is then endocytosed and binds to TRAM and TRIF. The end result is NFκB and MAPKs are activated and interferon type I is produced, due to activation of both MyD88 and TRIF.
Nuclear Receptors
As mentioned, lipid-soluble ligands can diffuse into cells on their own, without needing a cell membrane receptor. These ligands can bind to intracellular receptors, which then allows them to bind to nuclear DNA. These receptors are classified into four types, based on their mechanism. The two main types are:
- Type I. Type I receptors are found in the cytosol, and upon binding of ligand they homodimerize and translocate to the nucleus to bind to hormone response elements. Examples of these receptors include androgen receptors and glucocorticoid receptors.
- Type II. These receptors are always found in the nucleus, and form heterodimers with RXR. When ligand binds, corepressors are removed and coactivators bind, allowing transcription of the target gene. Examples of these receptors included retinoic acid receptor, retinoid X receptor and thyroid hormone receptor. Another example is peroxisome proliferator-activated receptor, which binds prostaglandins and leukotrienes to mediate their effects.
Other Pathways
- The Hippo pathway is involved in controlling organ size, by regulating cell proliferation and apoptosis. Mutations in Hippo lead to an overgrowth of the organ, making the animal “hippopotamus-like”.
- VEGFR has tyrosine kinase activity, and is able to activate multiple pathways with the goal of promoting angiogenesis:
- PI3K/Akt to promote cell survival.
- Ras/Raf/MEK/ERK to increase cell proliferation.
- Phospholipase C to increase cell proliferation, produce nitric oxide and prostaglandins.
Comparison Table
| Pathway | Receptor | Receptor Ligand | Secondary Signalling | Transcription Factor | Outcome |
|---|---|---|---|---|---|
| Akt/PKB | Receptor tyrosine kinase GPCR | Growth factors | PI3K -> PIP3 -> Akt | Akt | Cell survival and proliferation |
| JAK/STAT | Cytokine receptors | Cytokines | STATs | Dimerized STATs | Leukocyte development and activation Cell division |
| Ras/Raf/MEK/ERK | Receptor tyrosine kinase | Growth factors | Ras -> Raf -> MEK -> ERK | ERK | Cell proliferation |
| TGF-beta | TGF-beta type II receptor | TGF-beta | SMAD2/3 -> SMAD4 | Complexed SMAD2/3/4 | Cell cycle arrest Apoptosis |
| cAMP | GPCR | Various | Adenylyl cyclase -> ATP converted to cAMP -> protein kinase A | Protein kinase A | Glucose and lipid metabolism |
| Phospholipase C | GPCR | Various | PIP2 -> DAG and IP3 -> calcium channel and protein kinase C activation | Protein kinase C | Membrane structure Transcription Immune responses Cell growth |
| Frizzled | GPCR | Wnt | Dishevelled -> APC and Axin complex disrupted -> free beta-catenin | Beta-catenin | Cell survival Cell proliferation |
| Patched | Patched-1 | Sonic hedgehog | Inhibits Smoothened -> GLI | GLI | Angiogenesis Cell growth Anti-apoptosis |
| Notch | Notch | Transmembrane proteins on neighbouring cell | Cleaves Notch | Intracellular Notch | Angiogenesis |
| Toll-Like | TLR | PAMPs | MyD88 (usually) -> kinases | NFκB MAPK | Cellular activation Anti-apoptosis |
Zachary JF. Pathologic Basis of Veterinary Disease, Sixth Edition.
Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease, Tenth Edition.
Murphy KP, Janeway CA, Travers P et al. Janeway’s Immunobiology, Eighth Edition.